
Synopsis
Synopsis
0
CEP/COS
0
EU WC
0
NDC API
0
VMF
0
South Africa
DRUG PRODUCT COMPOSITIONS
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
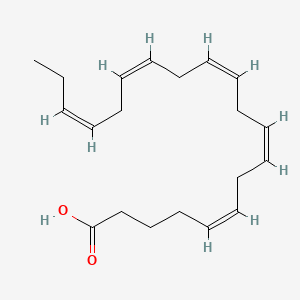

1. 5,8,11,14,17-eicosapentaenoic Acid
2. 5,8,11,14,17-icosapentaenoic Acid
3. Acid, Eicosapentanoic
4. Eicosapentanoic Acid
5. Icosapent
6. Omega 3 Eicosapentaenoic Acid
7. Omega-3-eicosapentaenoic Acid
8. Timnodonic Acid
1. Timnodonic Acid
2. Icosapent
3. 10417-94-4
4. Icosapentaenoic Acid
5. Epa
6. Cis-5,8,11,14,17-eicosapentaenoic Acid
7. (5z,8z,11z,14z,17z)-icosa-5,8,11,14,17-pentaenoic Acid
8. Icosapento
9. Icosapentum
10. 5,8,11,14,17-eicosapentaenoic Acid
11. Eicosapentaenoate
12. 5z,8z,11z,14z,17z-eicosapentaenoic Acid
13. (all-z)-5,8,11,14,17-eicosapentaenoic Acid
14. Icosapent [inn]
15. (5z,8z,11z,14z,17z)-5,8,11,14,17-eicosapentaenoic Acid
16. 5,8,11,14,17-icosapentaenoic Acid
17. Ropufa 70
18. Ccris 3279
19. Incromega E 7010sr
20. Aan7qov9ea
21. (5z,8z,11z,14z,17z)-icosapentaenoic Acid
22. (5z,8z,11z,14z,17z)-eicosapentaenoic Acid
23. Omega-3-carboxylic Acids
24. Epa 45g
25. Chembl460026
26. (5z,8z,11z,14z,17z)-eicosapentaenoate
27. All-cis-5,8,11,14,17-icosapentaenoic Acid
28. Chebi:28364
29. All-cis-5,8,11,14,17-eicosapentaenoic Acid
30. All-cis-icosa-5,8,11,14,17-pentaenoic Acid
31. Icosapent (inn)
32. Cis-delta(5,8,11,14,17)-eicosapentaenoic Acid
33. 5,8,11,14,17-eicosapentaenoic Acid, (all-z)-
34. All-cis-fatty Acid 20:5 Omega-3
35. Eicosapentaenoic Acid (c20:5 N3)
36. Epa;timnodonic Acid
37. Icosapentaenoate
38. (all-z)-delta5,8,11,14,17-eicosapentaenoic Acid
39. C20:5n-3,6,9,12,15
40. Cis-5,8,11,14,17-epa
41. Fa 20:5
42. Ncgc00161344-03
43. C20:5 (n-3)
44. Eicosapentaenoic Acid (20:5 N-3)
45. Eicosa-5z,8z,11z,14z,17z-pentaenoic Acid (20:5, N-3)
46. Miraxion
47. Eicosapentanoic Acid
48. Eye Q
49. Eye-q
50. 5,8,11,14,17-eicosapentaenoic Acid, (5z,8z,11z,14z,17z)-
51. Epa [drug]
52. Unii-aan7qov9ea
53. 1553-41-9
54. Icosapentum [inn-latin]
55. Icosapento [inn-spanish]
56. (5z,8z,11z,14z,17z)-icosapentaenoate
57. Timnodonate
58. 3gwx
59. All Cis-5,8,11,14,17-eicosapentaenoic Acid
60. Mfcd00065716
61. All-cis-icosapentaenoate
62. All-cis-icosapentaenoic Acid
63. Dsstox_cid_21023
64. Dsstox_rid_79612
65. Dsstox_gsid_41023
66. Schembl20469
67. Bspbio_001328
68. Bml3-b01
69. Gtpl3362
70. Dtxsid9041023
71. Eicosapentaenoic Acid [mi]
72. Hms1361c10
73. Hms1791c10
74. Hms1989c10
75. Hms3402c10
76. Hms3649d19
77. Hy-b0660
78. Zinc4474603
79. 5,8,11,14,17-icosapentaenoate
80. Eicosapentaenoic Acid [inci]
81. Tox21_111991
82. 5,8,11,14,17-eicosapentaenoate
83. Bdbm50242349
84. Eicosapentaenoic Acid [vandf]
85. Lmfa01030759
86. S6476
87. Eicosapentaenoic Acid [mart.]
88. Akos027470327
89. Eicosapentaenoic Acid [usp-rs]
90. Eicosapentaenoic Acid [who-dd]
91. Ccg-207957
92. Ccg-208136
93. Db00159
94. Cis-5,8,11,14,17-eicosapentaenoate
95. Idi1_033798
96. Ncgc00161344-01
97. Ncgc00161344-02
98. Ncgc00161344-04
99. Ncgc00161344-07
100. 5z,8z,11z,14z,17z-eicosapentaenoate
101. Ac-31072
102. As-53730
103. Cas-10417-94-4
104. E0441
105. 5,8,11,14,17-eicosapentaenoic Acid (6ci)
106. All Cis-5,8,11,14,17-icosapentaenoic Acid
107. C06428
108. D08061
109. P16966
110. (all-cis)-5,8,11,14,17-eicosapentaenoic Acid
111. Eicosapentaenoic Acid (epa) (c20:5 N3)
112. L001256
113. Q409990
114. Sr-01000946647
115. Fa(20:5(5z,8z,11z,14z,17z))
116. J-001125
117. Sr-01000946647-1
118. Z,z,z,z,z-eicosa-5,8,11,14,17-pentaenoic Acid
119. (z,z,z,z,z)-5,8,11,14,17-eicosapentaenoic Acid
120. Brd-k47192521-001-02-1
121. Cis-5,8,11,14,17-eicosapentaenoic Acid, >=99%
122. 7f8bf016-b146-4f72-a52e-b9298ba3a9ab
123. C20h30o2 (cis-5,8,11,14,17-eicosapentaenoic Acid)
124. Eicosapentaenoic Acid, 5,8,11,14,17-(z,z,z,z,z)-
125. 5,8,11,14,17-eicosapentaenoic Acid, (all-z)- (8ci)
126. Cis-5,8,11,14,17-eicosapentaenoic Acid, >=85%, Liquid
127. Cis-5,8,11,14,17-eicosapentaenoic Acid, Analytical Standard
128. (5z,8z,11 Z,14z,17z)-icosa-5,8,11,14,17-pentaenoic Acid
129. (5z,8z,11z,14z,17z)-eicosa-5,8,11,14,17-pentaenoic Acid
130. Cis, Cis, Cis, Cis, Cis-eicosa-5,8,11,14,17-pentaenoic Acid
131. 5,8,11,14,17-eicosapentaenoic Acid, (5z,8z,11z,14z,17z)- (9ci)
132. Eicosapentaenoic Acid (epa) (c20:5) (constituent Of Krill Oil) [dsc]
133. Cis-5,8,11,14,17-eicosapentaenoic Acid, 500 Mug/ml In Ethanol, Certified Reference Material
| Molecular Weight | 302.5 g/mol |
|---|---|
| Molecular Formula | C20H30O2 |
| XLogP3 | 5.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 13 |
| Exact Mass | 302.224580195 g/mol |
| Monoisotopic Mass | 302.224580195 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 398 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 5 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
EPA can be used for lowering elevated triglycerides in those who are hyperglyceridemic. In addition, EPA may play a therapeutic role in patients with cystic fibrosis by reducing disease severity and may play a similar role in type 2 diabetics in slowing the progression of diabetic nephropathy.
FDA Label
OM3-CA is indicated as an adjunct to diet to reduce triglycerides levels in adults patients with severe hypertriglyceridemia (>500 mg/dL). The patients involved in this treatment should be laced with an appropriate lipid-lowering diet. Hypertriglyceridemia is defined as an elevated plasma triglyceride concentration. It is usually correlated to other secondary conditions such as poor diet, alcohol use, obesity, metabolic syndrome and type 2 diabetes.
FDA Label
Treatment of Familial Adenomatous Polyposis
Treatment of dyslipidaemia
Eicosanoids are chemical messengers derived from 20-carbon polyunsaturated fatty acids that play critical roles in immune and inflammatory responses. Both 20-carbon omega-6 fatty acids (arachidonic acid) and 20-carbon omega-3 fatty acids (EPA) can be found in cell membranes. During an inflammatory response, arachidonic acid and EPA are metabolized by enzymes known as cyclooxygenases and lipoxygenases to form eicosanoids. Increasing omega-3 fatty acid intake increases the EPA content of cell membranes and decreases the arachidonic acid content, resulting in higher proportions of eicosanoids derived from EPA. Physiologic responses to arachidonic acid-derived eicosanoids differ from responses to EPA-derived eicosanoids. In general, eicosanoids derived from EPA are less potent inducers of inflammation, blood vessel constriction, and clotting than eicosanoids derived from arachidonic acid.
OM3-CA is very effective in reducing triglyceride levels. After 14 days of treatment, it is possible to observe even a 21% reduction. The reduction of the triglycerides could reach even to 25% in cases with the maximal used concentration of 4 g.
Absorption
When compared to omega-3 -acid ethyl esters, OM3-CA present a 4-fold higher bioavailability. OM3-CA is absorbed directly in the small intestine and the maximal plasma concentration is reached between 4.5-5 hours after initial administration. The absorbed dosage is transferred to the general circulation via the lymphatic system and distributed within tissues throughout the body. The absorption speed and extent is highly promoted by the bile. In preclinical studies performed in dogs, the Cmax, tmax and AUC were reported to be 15.1 mcg/ml, 24 hours and 1210.3 mcg.h/ml, respectively.
Route of Elimination
OM3-CA does not go under renal excretion. After the metabolism, all the dose is excreted as CO2 and water in the form of expelled air and the rest is excreted in feces.
Volume of Distribution
This pharmacokinetic property is not available.
Clearance
The registered clearance rate at steady-state is of 548 ml/h for eicosapentaenoic acid and 518 ml/h for docohexaenoic acid.
OM3-CA is metabolized in the liver following the normal fatty acid oxidation. Once absorbed, they are incorporated into triglycerides, cholesterol esters and phospholipids in tissues. The metabolism is marked by beta-oxidation followed by tricarboxylic acid cycle. It is reported that OM3-CA is an inhibitor of several enzymes such as CYP2C9, CYP2C19 and to a lesser extent to CYP1A2, CYP2E1, CYP3A4. It is thought that the metabolism of OM3-CA is mainly done by CYP3A and CYP4F3B.
Eicosapentaenoic acid has known human metabolites that include Juniperonic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The half-life of OM3-CA depends on the type of component in which for eicosapentaenoic acid it is estimated to be of approximately 4.7-10.8 hours while for docosahexaenoic acid is reported to be of about 7 hours. The half-life of baseline-adjusted at steady-state is of 36 and 46 hours respectively for eicosapentaenoic acid and docosahexaenoic acid.
The anti-inflammatory, antithrombotic and immunomodulatory actions of EPA is probably due to its role in eicosanoid physiology and biochemistry. Most eicosanoids are produced by the metabolism of omega-3 fatty acids, specifically, arachidonic acid. These eicosanoids, leukotriene B4 (LTB4) and thromboxane A2 (TXA2) stimulate leukocyte chemotaxis, platelet aggregation and vasoconstriction. They are thrombogenic and artherogenic. On the other hand, EPA is metabolized to leukotriene B5 (LTB5) and thromboxane A3 (TXA3), which are eicosanoids that promote vasodilation, inhibit platelet aggregation and leukocyte chemotaxis and are anti-artherogenic and anti-thrombotic. The triglyceride-lowering effect of EPA results from inhibition of lipogenesis and stimulation of fatty acid oxidation. Fatty acid oxidation of EPA occurs mainly in the mitochondria. EPA is a substrate for Prostaglandin-endoperoxide synthase 1 and 2. It also appears to affect the function and bind to the Carbohydrate responsive element binding protein (ChREBP) and to a fatty acid receptor (G-coupled receptor) known as GP40.
The reduction of the synthesis of triglycerides in the liver may be caused because the main components of OM3-CA, eicosapentaenoic acid, and docosahexaenoic acid, are poor substrates for the enzymes responsible for the synthesis of triglycerides. These two major components inhibit the esterification of other fatty acids. OM3-CA is also thought to enhance the clearance of triglycerides from the circulating very low-density lipoprotein particles by different potential effects such as inhibition of acyl-CoA:1,2-diacylglycerol acyltransferase, increase in mitochondrial and peroxisomal beta-oxidation in the liver, decrease lipogenesis in the liver and increase lipoprotein lipase activity.
 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2023-06-07
Pay. Date : 2023-06-05
DMF Number : 27182
Submission : 2013-05-23
Status : Active
Type : II
 Chunghwa provides cost-effective APIs & advanced intermediates with complete DMF or COS, ensuring quality & reliable production.
Chunghwa provides cost-effective APIs & advanced intermediates with complete DMF or COS, ensuring quality & reliable production.

GDUFA
DMF Review : Reviewed
Rev. Date : 2013-04-01
Pay. Date : 2012-11-20
DMF Number : 26271
Submission : 2012-08-01
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37763
Submission : 2022-12-11
Status : Active
Type : II



GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39040
Submission : 2023-10-25
Status : Active
Type : II



GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 27998
Submission : 2014-02-10
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2022-07-25
Pay. Date : 2022-06-10
DMF Number : 36822
Submission : 2022-06-07
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2020-11-02
Pay. Date : 2020-10-20
DMF Number : 23911
Submission : 2010-07-07
Status : Active
Type : II

Registration Number : 223MF10118
Registrant's Address : 4th and 5th Floors, 2 Stockport Exchange, Railway Road, Stockport, SK1 3GG, UK
Initial Date of Registration : 2011-07-25
Latest Date of Registration :



GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15062
Submission : 2000-09-29
Status : Active
Type : II

Registration Number : 217MF11111
Registrant's Address : 1-25 Kanda Nishikicho, Chiyoda-ku, Tokyo
Initial Date of Registration : 2005-12-05
Latest Date of Registration :


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 22474
Submission : 2009-01-23
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 34019
Submission : 2019-09-16
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
93
PharmaCompass offers a list of Icosapent Ethyl API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Icosapent Ethyl manufacturer or Icosapent Ethyl supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Icosapent Ethyl manufacturer or Icosapent Ethyl supplier.
PharmaCompass also assists you with knowing the Icosapent Ethyl API Price utilized in the formulation of products. Icosapent Ethyl API Price is not always fixed or binding as the Icosapent Ethyl Price is obtained through a variety of data sources. The Icosapent Ethyl Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Eicosapentaenoic Acid Ethyl Ester manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Eicosapentaenoic Acid Ethyl Ester, including repackagers and relabelers. The FDA regulates Eicosapentaenoic Acid Ethyl Ester manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Eicosapentaenoic Acid Ethyl Ester API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Eicosapentaenoic Acid Ethyl Ester manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Eicosapentaenoic Acid Ethyl Ester supplier is an individual or a company that provides Eicosapentaenoic Acid Ethyl Ester active pharmaceutical ingredient (API) or Eicosapentaenoic Acid Ethyl Ester finished formulations upon request. The Eicosapentaenoic Acid Ethyl Ester suppliers may include Eicosapentaenoic Acid Ethyl Ester API manufacturers, exporters, distributors and traders.
click here to find a list of Eicosapentaenoic Acid Ethyl Ester suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Eicosapentaenoic Acid Ethyl Ester DMF (Drug Master File) is a document detailing the whole manufacturing process of Eicosapentaenoic Acid Ethyl Ester active pharmaceutical ingredient (API) in detail. Different forms of Eicosapentaenoic Acid Ethyl Ester DMFs exist exist since differing nations have different regulations, such as Eicosapentaenoic Acid Ethyl Ester USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Eicosapentaenoic Acid Ethyl Ester DMF submitted to regulatory agencies in the US is known as a USDMF. Eicosapentaenoic Acid Ethyl Ester USDMF includes data on Eicosapentaenoic Acid Ethyl Ester's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Eicosapentaenoic Acid Ethyl Ester USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Eicosapentaenoic Acid Ethyl Ester suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Eicosapentaenoic Acid Ethyl Ester Drug Master File in Japan (Eicosapentaenoic Acid Ethyl Ester JDMF) empowers Eicosapentaenoic Acid Ethyl Ester API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Eicosapentaenoic Acid Ethyl Ester JDMF during the approval evaluation for pharmaceutical products. At the time of Eicosapentaenoic Acid Ethyl Ester JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Eicosapentaenoic Acid Ethyl Ester suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Eicosapentaenoic Acid Ethyl Ester Drug Master File in Korea (Eicosapentaenoic Acid Ethyl Ester KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Eicosapentaenoic Acid Ethyl Ester. The MFDS reviews the Eicosapentaenoic Acid Ethyl Ester KDMF as part of the drug registration process and uses the information provided in the Eicosapentaenoic Acid Ethyl Ester KDMF to evaluate the safety and efficacy of the drug.
After submitting a Eicosapentaenoic Acid Ethyl Ester KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Eicosapentaenoic Acid Ethyl Ester API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Eicosapentaenoic Acid Ethyl Ester suppliers with KDMF on PharmaCompass.
Eicosapentaenoic Acid Ethyl Ester Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Eicosapentaenoic Acid Ethyl Ester GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Eicosapentaenoic Acid Ethyl Ester GMP manufacturer or Eicosapentaenoic Acid Ethyl Ester GMP API supplier for your needs.
A Eicosapentaenoic Acid Ethyl Ester CoA (Certificate of Analysis) is a formal document that attests to Eicosapentaenoic Acid Ethyl Ester's compliance with Eicosapentaenoic Acid Ethyl Ester specifications and serves as a tool for batch-level quality control.
Eicosapentaenoic Acid Ethyl Ester CoA mostly includes findings from lab analyses of a specific batch. For each Eicosapentaenoic Acid Ethyl Ester CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Eicosapentaenoic Acid Ethyl Ester may be tested according to a variety of international standards, such as European Pharmacopoeia (Eicosapentaenoic Acid Ethyl Ester EP), Eicosapentaenoic Acid Ethyl Ester JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Eicosapentaenoic Acid Ethyl Ester USP).

