
Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
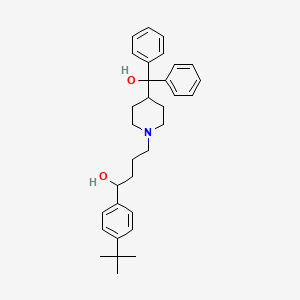

1. Alpha-(4-(1,1-dimethylethyl)phenyl)-4-(hydroxydiphenylmethyl)-1-piperdinebutanol
2. Balkis Saft Spezial
3. Cyater
4. Hisfedin
5. Rapidal
6. Rmi 9918
7. Rmi-9918
8. Rmi9918
9. Seldane
10. Teldane
11. Terfedura
12. Terfemundin
13. Terfenadin Al
14. Terfenadin Heumann
15. Terfenadin Ratiopharm
16. Terfenadin Stada
17. Terfenadin Von Ct
18. Terfenadin-ratiopharm
19. Terfenidine
20. Ternadin
21. Triludan
1. 50679-08-8
2. Seldane
3. Ternadin
4. Triludan
5. Teldane
6. Allerplus
7. Teldanex
8. Aldaban
9. Terdin
10. Terfenadina
11. Rmi 9918
12. Rmi-9918
13. 1-(4-(tert-butyl)phenyl)-4-(4-(hydroxydiphenylmethyl)piperidin-1-yl)butan-1-ol
14. 1-(4-tert-butylphenyl)-4-[4-[hydroxy(diphenyl)methyl]piperidin-1-yl]butan-1-ol
15. Alpha-[4-(1,1-dimethylethyl)phenyl]-4-(hydroxydiphenylmethyl)-1-piperidinebutanol
16. Mdl-9918
17. Nsc-665802
18. Nsc-758627
19. Chembl17157
20. 1-piperidinebutanol, .alpha.-[4-(1,1-dimethylethyl)phenyl]-4-(hydroxydiphenylmethyl)-
21. Mls000028499
22. 7ba5g9y06q
23. Chebi:9453
24. Cyater
25. Terfen
26. Alpha-(4-(1,1-dimethylethyl)phenyl)-4-(hydroxydiphenylmethyl)-1-piperidinebutanol
27. Ncgc00016064-04
28. Ncgc00016064-06
29. Terfenadinum
30. Smr000058260
31. Terfex
32. Dsstox_cid_3642
33. 1-(4-tert-butylphenyl)-4-[4-(hydroxydiphenylmethyl)piperidin-1-yl]butan-1-ol
34. 1-(4-(tert-butyl)phenyl)-4-(4-(hydroxydiphenylmethyl)-piperidin-1-yl)butan-1-ol
35. Dsstox_rid_77124
36. Dsstox_gsid_23642
37. Terfenadinum [inn-latin]
38. Terfenadina [inn-spanish]
39. .alpha.-(p-tert-butylphenyl)-4-(hydroxydiphenylmethyl)-1-piperidinebutanol
40. 1-(4-tert-butylphenyl)-4-(4-(hydroxydiphenylmethyl)piperidin-1-yl)butan-1-ol
41. Mdl 9918
42. Alpha-(4-[1,1-dimethylethyl]phenyl)-4-[hydroxydiphenylmethyl]-1-piperidinebutanol
43. Seldane (tn)
44. Hsdb 6508
45. Sr-01000000104
46. Einecs 256-710-8
47. Mfcd00079622
48. Brn 5857899
49. Teraenadine
50. Unii-7ba5g9y06q
51. Terfenadin
52. Terfenadine (jan/usan/inn)
53. Terfenadine,(s)
54. (-)-terfenadine
55. 1-piperidinebutanol, .alpha.-(4-(1,1-dimethylethyl)phenyl)-4-(hydroxydiphenylmethyl)-
56. Alpha-(p-tert-butylphenyl)-4-(hydroxydiphenylmethyl)-1-piperidinebutanol
57. Prestwick_460
58. 1-(4-tert-butylphenyl)-4-(4-(alpha-hydroxybenzhydryl)piperidino)-butan-1-ol
59. 1-(p-tert-butylphenyl)-4-(4'-(alpha-hydroxydiphenylmethyl)-1'-piperidyl)butanol
60. Cas-50679-08-8
61. 1-piperidinebutanol, Alpha-(4-(1,1-dimethylethyl)phenyl)-4-(hydroxydiphenylmethyl)-
62. Terfenadine [usan:usp:inn:ban:jan]
63. Opera_id_334
64. Terfenadine [mi]
65. Prestwick0_000138
66. Prestwick1_000138
67. Prestwick2_000138
68. Prestwick3_000138
69. Terfenadine [inn]
70. Terfenadine [jan]
71. (.+/-.)-terfenadine
72. Terfenadine [hsdb]
73. Terfenadine [usan]
74. T 9652
75. Terfenadine [vandf]
76. Schembl5152
77. Terfenadine [mart.]
78. Lopac0_001213
79. Oprea1_343139
80. Bspbio_000216
81. Terfenadine [who-dd]
82. Mls001148415
83. Divk1c_001011
84. Spbio_002155
85. Bpbio1_000238
86. Gtpl2608
87. Dtxsid2023642
88. Hms503k03
89. Kbio1_001011
90. Mdl-991
91. Terfenadine [ep Impurity]
92. Ninds_001011
93. Hms1568k18
94. Hms2089i14
95. Hms2093i11
96. Hms2095k18
97. Hms2231n07
98. Hms3263d08
99. Hms3373j07
100. Hms3712k18
101. Pharmakon1600-01503708
102. Terfenadine [ep Monograph]
103. Hy-b1193
104. Tox21_110295
105. Tox21_110296
106. Tox21_501213
107. Ac-928
108. Bdbm50017376
109. Nsc665802
110. Nsc758627
111. S4353
112. Akos015962091
113. Tox21_110295_1
114. Ccg-205287
115. Cs-4800
116. Db00342
117. Lp01213
118. Nsc 665802
119. Nsc 758627
120. Sdccgsbi-0051180.p004
121. Terfenadine 100 Microg/ml In Methanol
122. 1-(4-tert-butylphenyl)-4-[4-[hydroxy(diphenyl)methyl]-1-piperidyl]butan-1-ol
123. 1-[4-(1,1-dimethylethyl)phenyl]-4-{4-[hydroxy(diphenyl)methyl]piperidin-1-yl}butan-1-ol
124. Idi1_001011
125. Ncgc00016064-03
126. Ncgc00016064-05
127. Ncgc00016064-08
128. Ncgc00016064-09
129. Ncgc00016064-12
130. Ncgc00016064-19
131. Ncgc00089746-02
132. Ncgc00089746-03
133. Ncgc00089746-04
134. Ncgc00261898-01
135. ( Inverted Exclamation Marka)-terfenadine
136. Ac-15791
137. As-75636
138. Bt166253
139. Nci60_022784
140. Sbi-0051180.p003
141. Db-051821
142. Ab00052367
143. Eu-0101213
144. Ft-0630637
145. Sw196677-3
146. T3977
147. Bim-0051180.0001
148. C07463
149. D00521
150. D82079
151. Ab00052367-16
152. Ab00052367_17
153. Ab00052367_18
154. A828247
155. L000888
156. Q417909
157. ( Inverted Exclamation Marka)-terfenadine;mdl-991
158. ( Inverted Exclamation Marka)-terfenadine;mdl-9918
159. Sr-01000000104-2
160. Sr-01000000104-4
161. Sr-01000000104-7
162. Brd-a06352418-001-03-6
163. Brd-a06352418-001-15-0
164. Terfenadine, European Pharmacopoeia (ep) Reference Standard
165. Terfenadine, United States Pharmacopeia (usp) Reference Standard
166. (+/-)1-(4-tert-butylphenyl)-4-(4-(hydroxydiphenylmethyl)piperidin-1-yl)butan-1-ol
167. (rs)-1-(4-tert-butylphenyl)-4-{4-[hydroxy(diphenyl)methyl]piperidin-1-yl}-butan-1-ol
168. .alpha.-(4-(1,1-dimethylethyl)phenyl)-4-(hydroxydiphenyl-methyl)-1-piperidine Butanol
169. .alpha.-(4-(1,1-dimethylethyl)phenyl)-4-(hydroxydiphenylmethyl)-1-piperidinebutanol
170. 1-(4-tert-butyl-phenyl)-4-[4-(hydroxy-diphenyl-methyl)-piperidin-1-yl]-butan-1-ol
171. 1-(4-tert-butylphenyl)-4-(4-[hydroxy(diphenyl)methyl]-1-piperidinyl)-1-butanol #
172. 1-(4-tert-butylphenyl)-4-[4-[hydroxy-di(phenyl)methyl]piperidin-1-yl]butan-1-ol
173. A-[4-(1,1-dimethylethyl)phenyl]-4-(hydroxydiphenylmethyl)-1-piperidinebutanol
174. Alpha-[4-(1,1-dmethylethyl)phenyl]-4-(hydroxydiphenylmethyl)-1-piperidinebutanol
175. 1-(4-tert-butyl-phenyl)-4-[4-(hydroxy-diphenyl-methyl)-piperidin-1-yl]-butan-1-ol(terfenadine)
| Molecular Weight | 471.7 g/mol |
|---|---|
| Molecular Formula | C32H41NO2 |
| XLogP3 | 6.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 9 |
| Exact Mass | 471.313729551 g/mol |
| Monoisotopic Mass | 471.313729551 g/mol |
| Topological Polar Surface Area | 43.7 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 582 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Allergic Agents; Anti-Asthmatic Agents; Histamine H1 Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Antihistaminic
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1635
This study was a single center trial comparing the effects of the nonsedating antihistamine terfenadine, at a dose of 120 mg twice a day, with placebo in the treatment of rhinitis symptoms associated with the common cold. Forty-nine subjects were treated with terfenadine, 120 mg twice each day, and 48 subjects were treated with placebo twice each day for four or five days. Evaluations by both subjects and physicians suggest that terfenadine at 120 mg given twice daily marginally improved sneezing and total symptom scores at day 4. When comparing terfenadine to placebo, neither the symptoms nor signs of the common cold improved in a clinically or statistically significant manner. Terfenadine was well tolerated and had a low incidence of side effects. Terfenadine was found to be ineffective in the treatment of the signs and symptoms of the common cold.
PMID:1750722 Berkowitz RB, Tinkelman DG; Ann Allergy 67 (6): 593-7 (1991)
Results of a double blind, randomized, placebo controlled, parallel study in 37 patients indicate that terfenadine, 60 mg twice a day, is significantly more effective than placebo and as effective as hydroxyzine, 25 mg four times a day in the treatment of chronic idiopathic urticaria without causing the somnolence that was associated with the use of hydroxyzine.
PMID:2574555 Boggs PB et al; Ann Allergy 63 (6, Pt 2): 616-20 (1989)
For more Therapeutic Uses (Complete) data for TERFENADINE (10 total), please visit the HSDB record page.
Hoechst Marion Roussel, the manufacturer of Seldane and Seldane-D announced that as of February 1, 1998, it will withdraw all single-entity (Seldane) and fixed combination (Seldane-D) preparations of terfenadine from the US market. This announcement was preceded by a declaration of the US Food and Drug administration (FDA) in January 1997 of the agency's intent to withdraw all terfenadine preparations from the US since continued marketing of terfenadine, which interacts with numerous drugs and is potentially cardiotoxic, was no longer necessary because of the availability of a single-entity preparation of fexofenadine (Allegra), the pharmacologically active metabolite of terfenadine that does not share the cardiotoxic and drug interaction potentials of the parent drug. As a result of fexofenadine availability (both as a single entity and fixed combination preparation) as alternatives, Hoechst Marion Roussel concluded that the benefits of continued availability of terfenadine preparations did not outweigh the risks.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 38
The most frequent adverse effects reported with terfenadine are sedation (eg, drowsiness, tiredness, sleepiness, fatigue) and headache, which occur in about 5-16% of patients receiving the drug. Other less frequent adverse nervous system effects include dizziness, nervousness, and weakness. Mental depression, anxiety, malaise, agitation, euphoria, fainting sensation, floating feeling, fear of dying, tingling (eg, of the extremities), insomnia, paresthesia, tremor, decreased concentrating ability, confusion, and nightmares also have occurred. Irritability, incoordination, and vertigo have been reported rarely, and seizures and exacerbation of an underlying seizure disorder also have been reported rarely, usually in association with overdosage of the drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 40
Adverse GI effects reportedly occurring in about 5-8% of patients receiving terfenadine Include abdominal distress, nausea, vomiting, and a change in bowel habits (eg, constipation, diarrhea). Increased appetite and weight gain also have been reported.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 40
Dry mouth, nose, throat, and/or lips; cough; sore throat; and epistaxis occur in less than 5% of patients receiving terfenadine.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 40
For more Drug Warnings (Complete) data for TERFENADINE (16 total), please visit the HSDB record page.
For the treatment of allergic rhinitis, hay fever, and allergic skin disorders.
Terfenadine, an H1-receptor antagonist antihistamine, is similar in structure to astemizole and haloperidol, a butyrophenone antipsychotic. The active metabolite of terfenadine is fexofenadine.
Histamine H1 Antagonists, Non-Sedating
A class of non-sedating drugs that bind to but do not activate histamine receptors (DRUG INVERSE AGONISM), thereby blocking the actions of histamine or histamine agonists. These antihistamines represent a heterogenous group of compounds with differing chemical structures, adverse effects, distribution, and metabolism. Compared to the early (first generation) antihistamines, these non-sedating antihistamines have greater receptor specificity, lower penetration of BLOOD-BRAIN BARRIER, and are less likely to cause drowsiness or psychomotor impairment. (See all compounds classified as Histamine H1 Antagonists, Non-Sedating.)
R - Respiratory system
R06 - Antihistamines for systemic use
R06A - Antihistamines for systemic use
R06AX - Other antihistamines for systemic use
R06AX12 - Terfenadine
Absorption
On the basis of a mass balance study using 14C labeled terfenadine the oral absorption of terfenadine was estimated to be at least 70%
Although at least 70% of an oral dose of terfenadine is rapidly absorbed from the GI tract following oral administration, the drug undergoes extensive (99%) first-pass metabolism in the liver and GI tract, with minimal (10 ng/mL or less)amounts of an orally administered dose of the drug generally appearing to reach systemic circulation unchanged in healthy individuals. In some cases, increased plasma terfenadine concentrations (exceeding 10 ng/mL) following oral administration of the drug were reported in apparently healthy individuals with no identifiable risk for systemic accumulation of unchanged drug; ... Considerable interindividual variations (up to five-fold) in peak plasma concentrations have been reported with the same oral dose of terfenadine, possibly resulting from interindividual differences in first-pass metabolism and/or enterohepatic circulation of the drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 38
The absolute bioavailability of oral terfenadine is not known. When administered orally, terfenadine exhibits linear pharmacokinetics up to doses of 180 mg.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 38
Food may effect the rate slightly but does not appear to effect the extent of GI absorption of terfenadine.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 38
Following oral administration of a single 60-mg terfenadine dose (as tablet or suspension, peak plasma concentrations of the drug occur at about 1-2 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 39
For more Absorption, Distribution and Excretion (Complete) data for TERFENADINE (17 total), please visit the HSDB record page.
Hepatic
Although the exact metabolic fate of terfenadine is not clearly established, the drug is extensively metabolized in the liver by cytochrome P-450 microsomal enzyme system including CYP3A4 and to a lesser extent in the GI mucosa by CYP3A, principally via oxidation of the terminal methyl group to fexofenadine and via N-dealkylation of the substituted butanol side chain to a piperidine carbinol derivative (alpha,alpha-diphenyl-4-piperidinemethanol). Small amounts of other hydroxylated metabolites also have been detected, but their exact structures have not been elucidated.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 39
It has been suggested that fexofenadine, the main metabolite of terfenadine, may be responsible for the antihistaminic effect of terfenadine since only minimal amounts (10 ng/mL or less) of unchanged drug usually are detected in plasma following oral administration of terfenadine in healthy individuals. The piperidine carbinol derivative lacks both in vivo and in vitro antihistaminic activity.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 39
Terfenadine (Seldane) undergoes extensive metabolism to form azacyclonol and terfenadine alcohol. Terfenadine alcohol is subsequently metabolized to azacyclonol and terfenadine acid. Although testosterone 6 beta-hydroxylation (CYP3A(4)) has been shown to be the principal enzyme involved in the first step in terfenadine's biotransformation (formation of azacyclonol and terfenadine alcohol), the enzymes catalyzing the subsequent metabolic steps in the conversion of terfenadine alcohol to azacyclonol and terfenadine acid have not been identified. The purpose of these studies was to determine the role of cytochrome P450 isoforms in the biotransformation of terfenadine and terfenadine alcohol. To this end, both terfenadine and its alcohol were incubated with 10 individual human liver microsomal samples that have been characterized for major isozyme activities. The metabolites and parent drugs were quantified by HPLC. The formation of azacyclonol and terfenadine alcohol from terfenadine is confirmed to be catalyzed predominantly by CYP3A(4) isozyme, and the ratio of the rate of terfenadine alcohol formation to that of azacyclonol is 3:1. Involvement of the CYP3A(4) in terfenadine metabolism was further confirmed by the following studies: a) inhibition of terfenadine alcohol formation by ketoconazole and troleandomycin, two specific inhibitors of CYP3A(4), and b) time course of terfenadine alcohol formation by cloned human CYP3A(4). When terfenadine alcohol was used as substrate, both the terfenadine acid and azacyclonol formation were also catalyzed by CYP3A(4) isozyme. However, the rate of formation of the terfenadine acid metabolite is almost 9 times faster than that of azacyclonol. The net ratio of terfenadine acid to azacyclonol is 2:1.
PMID:7587944 Ling KH et al; Drug Metab Dispos. 1995 Jun;23(6):631-6
3.5 hours
Following multiple oral dosing of 60 mg of terfenadine twice daily, steady-state mean elimination half-lives of unchanged terfenadine and the carboxylic acid metabolite (fexofenadine) were 16.4 and 20.2 hours, respectively.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 39
Elimination half-life: 20.3 hr
US Pharmacopeial Convention; US Pharmacopeia Dispensing Information (USP DI); Drug Information for the Health Care Professional 12th ed, V.I p.397 (1992)
Terfenadine competes with histamine for binding at H1-receptor sites in the GI tract, uterus, large blood vessels, and bronchial muscle. This reversible binding of terfenadine to H1-receptors suppresses the formation of edema, flare, and pruritus resulting from histaminic activity. As the drug does not readily cross the blood-brain barrier, CNS depression is minimal.
... Terfenadine appears to have a dual effect on histamine H1-receptors. In vitro studies indicate that terfenadine competitively antagonizes the actions of histamine at concentrations of 15-47 ng/mL, while a relatively irreversible antagonism occurs at higher concentrations (ie, 150-470 ng/mL). Experimental evidence indicates that the drug exhibits a specific and selective antagonism of histamine H1-receptors and that the drug slowly binds to the H1-receptor and forms a stable complex from which it subsequently slowly dissociates. These finding suggest that the prolonged and generally irreversible nature of terfenadin's antagonism of histamine results principally from the drugs slow dissociation from the H1-receptors.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 38
Unlike many other antihistamines, terfenadine does not possess appreciable anticholinergic or antiserotonergic effects at usual antihistaminic doses in pharmacologic studies. However, in clinical trials there was no difference in the frequency of anticholinergic-like effects (eg, dryness of the nose, mouth, throat and/or lips)observed with terfenadine or other antihistamines (ie, chlopheniramine, clemastine, dexchlorpheniramine). Terfenadine also does not exhibit any appreciable alpha or beta-adrenergic blocking activity or histamine H2-receptor antagonism.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 38
Terfenadine has increased urinary bladder capacity in individuals with normal bladder function and in some patients with neurogenic bladder and overactive detrusor muscle function, probably via a histamine H1-antagonist effect on the detrusor muscle; this effect appears to vary diurnally, being maximal at night.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 38
The mechanism of the cardiotoxic effects of certain "nonsedating" antihistamines including terfenadine currently is not understood, and would appear to be contrary to what would be expected from studies on cardiac histamine H1-receptors; therefore, the possibility that H3-receptors (mediating a regulatory feedback mechanism) may be involved has been suggested. Limited evidence from animal models using terfenadine suggests that the cardiotoxic effects of the drug may result at least in part from blockade of the potassium channel involved in repolarization of cardiac cells (ie, blockade of the delayed rectifier potassium current IK). In some animal studies using fexofenadine, no blockade of the potassium channel involved in repolarization of cardiac cells was observed which may indicate a lack of fexofenadine-induced cardiotoxicity. In addition,in in vitro studies using fexofenadine, no effect was observed on delayed rectifier potassium channel cloned from human heart at fexofenadine concentrations up to 1.0X10-5M. Unlike with other antihistamines, anticholinergic and/or local anesthetic effects appear to be unlikely cause of the cardiac effects of certain "nonsedating" antihistamines, including terfenadine.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 40
Basophils in mononuclear cell populations were challenged with allergens, anti-immunoglobulin E (anti-IgE), C5a or formyl-methyl-leucyl-phenylalanine (FMLP), with or without a short pre-incubation with interleukin-3 (IL-3), in the presence of increasing concentrations of terfenadine. At doses of 0.1-1 ug/mL, terfenadine inhibits histamine release and generation of the sulfidoleukotrienes, leukotriene C4, D4 and E4 in basophils challenged with an IgE-dependent trigger. At concentrations above 10 ug/mL, however, terfenadine induces the histamine release but abolishes the formation of leukotrienes, and this may be due to a cytotoxic effect. In eosinophils, by contrast, terfenadine appears to inhibit the production of leukotrienes by eosinophils, triggered by FMLP only at concentrations above 10 ug/mL (which are toxic to basophils at least). In a double-blind, placebo-controlled study, 15 allergic patients were given skin challenges with specific allergen and with histamine, before and at 3 days, 2 and 4 weeks after treatment with terfenadine (120 mg/day for 3 days). The skin reactions were evaluated visually and followed kinetically by thermography. Terfenadine caused a significant decrease in both the immediate and late-phase reactions. Late-phase reactions to histamine were shown with thermography in some of the patients tested.
PMID:7686799 de Weck AL et al; Int Arch Allergy Immunol 101 (3): 326-32 (1993)
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
10
PharmaCompass offers a list of Terfenadine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Terfenadine manufacturer or Terfenadine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Terfenadine manufacturer or Terfenadine supplier.
PharmaCompass also assists you with knowing the Terfenadine API Price utilized in the formulation of products. Terfenadine API Price is not always fixed or binding as the Terfenadine Price is obtained through a variety of data sources. The Terfenadine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A DSSTox_CID_3642 manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of DSSTox_CID_3642, including repackagers and relabelers. The FDA regulates DSSTox_CID_3642 manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. DSSTox_CID_3642 API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of DSSTox_CID_3642 manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A DSSTox_CID_3642 supplier is an individual or a company that provides DSSTox_CID_3642 active pharmaceutical ingredient (API) or DSSTox_CID_3642 finished formulations upon request. The DSSTox_CID_3642 suppliers may include DSSTox_CID_3642 API manufacturers, exporters, distributors and traders.
click here to find a list of DSSTox_CID_3642 suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A DSSTox_CID_3642 DMF (Drug Master File) is a document detailing the whole manufacturing process of DSSTox_CID_3642 active pharmaceutical ingredient (API) in detail. Different forms of DSSTox_CID_3642 DMFs exist exist since differing nations have different regulations, such as DSSTox_CID_3642 USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A DSSTox_CID_3642 DMF submitted to regulatory agencies in the US is known as a USDMF. DSSTox_CID_3642 USDMF includes data on DSSTox_CID_3642's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The DSSTox_CID_3642 USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of DSSTox_CID_3642 suppliers with USDMF on PharmaCompass.
A DSSTox_CID_3642 CEP of the European Pharmacopoeia monograph is often referred to as a DSSTox_CID_3642 Certificate of Suitability (COS). The purpose of a DSSTox_CID_3642 CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of DSSTox_CID_3642 EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of DSSTox_CID_3642 to their clients by showing that a DSSTox_CID_3642 CEP has been issued for it. The manufacturer submits a DSSTox_CID_3642 CEP (COS) as part of the market authorization procedure, and it takes on the role of a DSSTox_CID_3642 CEP holder for the record. Additionally, the data presented in the DSSTox_CID_3642 CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the DSSTox_CID_3642 DMF.
A DSSTox_CID_3642 CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. DSSTox_CID_3642 CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of DSSTox_CID_3642 suppliers with CEP (COS) on PharmaCompass.
DSSTox_CID_3642 Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of DSSTox_CID_3642 GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right DSSTox_CID_3642 GMP manufacturer or DSSTox_CID_3642 GMP API supplier for your needs.
A DSSTox_CID_3642 CoA (Certificate of Analysis) is a formal document that attests to DSSTox_CID_3642's compliance with DSSTox_CID_3642 specifications and serves as a tool for batch-level quality control.
DSSTox_CID_3642 CoA mostly includes findings from lab analyses of a specific batch. For each DSSTox_CID_3642 CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
DSSTox_CID_3642 may be tested according to a variety of international standards, such as European Pharmacopoeia (DSSTox_CID_3642 EP), DSSTox_CID_3642 JP (Japanese Pharmacopeia) and the US Pharmacopoeia (DSSTox_CID_3642 USP).

