



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
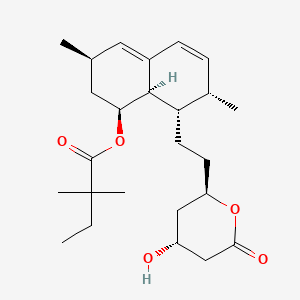

1. Mk 733
2. Mk-733
3. Mk733
4. Synvinolin
5. Zocor
1. 79902-63-9
2. Zocor
3. Synvinolin
4. Mk-733
5. Sinvacor
6. Denan
7. Lipex
8. Sivastin
9. Lodales
10. Simvastatine
11. Cholestat
12. Colemin
13. Simovil
14. Medipo
15. Pantok
16. Simvastatina
17. Simvastatinum
18. Velostatin
19. Zocord
20. Zorced
21. Simvastatin Lactone
22. Simvastatin (zocor)
23. Lipovas
24. Simcard
25. Simvacor
26. Simvoget
27. Rechol
28. Simlup
29. Mk-0733
30. (1s,3r,7s,8s,8ar)-8-(2-((2r,4r)-4-hydroxy-6-oxotetrahydro-2h-pyran-2-yl)ethyl)-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbutanoate
31. 2,2-dimethylbutyric Acid, 8-ester With (4r,6r)-6-(2-((1s,2s,6r,8s,8ar)-1,2,6,7,8,8a-hexahydro-8-hydroxy-2,6-dimethyl-1-naphthyl)ethyl)tetrahydro-4-hydroxy-2h-pyran-2-one
32. Mk 733
33. C10aa01
34. Agg2fn16ev
35. Nsc-758706
36. Chebi:9150
37. (1s,3r,7s,8s,8ar)-8-{2-[(2r,4r)-4-hydroxy-6-oxotetrahydro-2h-pyran-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbutanoate
38. [(1s,3r,7s,8s,8ar)-8-[2-[(2r,4r)-4-hydroxy-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] 2,2-dimethylbutanoate
39. Butanoic Acid, 2,2-dimethyl-, (1s,3r,7s,8s,8ar)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2r,4r)-tetrahydro-4-hydroxy-6-oxo-2h-pyran-2-yl]ethyl]-1-naphthalenyl Ester
40. Labistatin
41. Coledis
42. Corolin
43. Nivelipol
44. Rendapid
45. Vasotenal
46. Simvastatine [french]
47. Simvastatinum [latin]
48. Simvastatina [spanish]
49. Dsstox_cid_3581
50. Dsstox_rid_77090
51. Dsstox_gsid_23581
52. Butanoic Acid, 2,2-dimethyl-, (1s,3r,7s,8s,8ar)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-((2r,4r)-tetrahydro-4-hydroxy-6-oxo-2h-pyran-2-yl)ethyl)-1-naphthalenyl Ester
53. Zosta
54. Simvast Cr
55. Drg-0320
56. [(1s,3r,7s,8s,8ar)-8-[2-[(2r,4r)-4-hydroxy-6-oxo-tetrahydropyran-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] 2,2-dimethylbutanoate
57. Smr000718785
58. Mk 0733
59. Ccris 7558
60. Zocor (tn)
61. Hsdb 7208
62. Simvastatin & Primycin
63. Mk733
64. Sr-05000001894
65. Unii-agg2fn16ev
66. L 644128-000u
67. Brn 4768037
68. Kolestevan
69. Lipinorm
70. Modutrol
71. Simvotin
72. Sinvascor
73. Valemia
74. Eucor
75. Nor-vastina
76. Simvastatin,(s)
77. Simvastatin Predrug
78. (+)-simvastatin
79. Ncgc00016940-01
80. Inactive Simvastatin
81. Simvastatin [usan:usp:inn:ban]
82. Tnp00259
83. Prestwick_171
84. Simvastatin- Bio-x
85. Cas-79902-63-9
86. Ks-1113
87. Spectrum_001717
88. Specplus_000895
89. Simvastatin [mi]
90. Prestwick0_000865
91. Prestwick1_000865
92. Prestwick2_000865
93. Prestwick3_000865
94. Spectrum2_001671
95. Spectrum3_000669
96. Spectrum4_000632
97. Spectrum5_001428
98. Simvastatin [inn]
99. Simvastatin [jan]
100. Simvastatin [hsdb]
101. Simvastatin [usan]
102. Simvastatin [vandf]
103. Schembl2471
104. Chembl1064
105. Simvastatin [mart.]
106. Bspbio_000909
107. Bspbio_002337
108. Kbiogr_001244
109. Kbioss_002197
110. Simvastatin [usp-rs]
111. Simvastatin [who-dd]
112. Mls001304029
113. Mls001333077
114. Mls001333078
115. Mls002154038
116. Mls006011866
117. Bidd:gt0769
118. Divk1c_006991
119. Spectrum1504236
120. Spbio_001881
121. Spbio_002830
122. Bpbio1_001001
123. Gtpl2955
124. Simvastatin (jp17/usp/inn)
125. Simvastatin, Analytical Standard
126. Dtxsid0023581
127. Bcbcmap01_000007
128. Kbio1_001935
129. Kbio2_002197
130. Kbio2_004765
131. Kbio2_007333
132. Kbio3_001557
133. Rymzzmvnjrmudd-hgqwonqesa-
134. Simvastatin [orange Book]
135. Hms1570n11
136. Hms1922h13
137. Hms2089d12
138. Hms2093e06
139. Hms2097n11
140. Hms2231n22
141. Hms3259b12
142. Hms3412p08
143. Hms3676p08
144. Hms3714n11
145. Hms3884g10
146. Pharmakon1600-01504236
147. Simvastatin [ep Monograph]
148. Simcor Component Simvastatin
149. Simvastatin [usp Monograph]
150. Butanoic Acid, 2,2-dimethyl-, 1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-(tetrahydro-4-hydroxy-6-oxo-2h-pyran-2-yl)ethyl)-1-naphthalenyl Ester, (1s-(1.alpha.,3.alpha.,7.beta.,8.beta.(2s*,4s*),8a.beta.))-
151. Polycap Component Simvastatin
152. Vytorin Component Simvastatin
153. Zinc3780893
154. Tox21_110696
155. Tox21_300400
156. Bbl024390
157. Bdbm50139181
158. Ccg-39069
159. Nsc633782
160. Nsc758706
161. S1792
162. Stk801938
163. Akos005111006
164. Akos015842733
165. Simvastatin Component Of Simcor
166. Simvastatin, >=97% (hplc), Solid
167. Tox21_110696_1
168. Ac-1530
169. Db00641
170. Nc00719
171. Nsc 758706
172. Nsc-633782
173. Simvastatin Component Of Vytorin
174. Mrf-0000729
175. Ncgc00017324-01
176. Ncgc00017324-02
177. Ncgc00017324-03
178. Ncgc00017324-04
179. Ncgc00017324-05
180. Ncgc00017324-07
181. Ncgc00017324-08
182. Ncgc00017324-09
183. Ncgc00254418-01
184. 2,2-dimethylbutanoic Acid (1s,3r,7s,8s,8ar)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2r,4r)-tetrahydro-4-hydroxy-6-oxo-2h-pyran-2-yl]ethyl]-1-naphthalenyl Ester
185. Bs164407
186. Butanoic Acid, 2,2-dimethyl-, 1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-(tetrahydro-4-hydroxy-6-oxo-2h-pyran-2-yl)ethyl)-1-naphthalenyl Ester, (1s-(1alpha,3alpha,7beta,8beta(2s*,4s*),8abeta))-
187. Hy-17502
188. Sbi-0206773.p001
189. Simvastatin 100 Microg/ml In Acetonitrile
190. S0509
191. D00434
192. Ab00053395-07
193. Ab00053395-08
194. Ab00053395-10
195. Ab00053395_11
196. Ab00053395_13
197. 902s639
198. A839783
199. Q670131
200. Sr-05000001894-1
201. Sr-05000001894-2
202. Brd-k22134346-001-05-8
203. Brd-k22134346-001-11-6
204. Brd-k22134346-001-15-7
205. Z1741982918
206. Simvastatin, British Pharmacopoeia (bp) Reference Standard
207. Simvastatin, European Pharmacopoeia (ep) Reference Standard
208. Simvastatin, United States Pharmacopeia (usp) Reference Standard
209. Simvastatin, Pharmaceutical Secondary Standard; Certified Reference Material
210. Simvastatin For Peak Identification, European Pharmacopoeia (ep) Reference Standard
211. (1s,3r,7s,8s,8ar)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2r,4r)-tetrahydro-4-hydroxy-6-oxo-2h-pyran-2-yl]ethyl]-1-naphthalenyly-2,2-dimethyl Butanoate
212. (1s,3r,7s,8s,8ar)-8-(2-((2r,4r)-4-hydroxy-6-oxotetrahydro-2h-pyran-2-yl)ethyl)-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbu
213. (1s,7s,8s,8ar)-8-{2-[(2r,4r)-4-hydroxy-6-oxooxan-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbutanoate
| Molecular Weight | 418.6 g/mol |
|---|---|
| Molecular Formula | C25H38O5 |
| XLogP3 | 4.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Exact Mass | 418.27192431 g/mol |
| Monoisotopic Mass | 418.27192431 g/mol |
| Topological Polar Surface Area | 72.8 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 706 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Simvastatin |
| PubMed Health | Simvastatin (By mouth) |
| Drug Classes | Antihyperlipidemic |
| Drug Label | Simvastatin is a lipid-lowering agent that is derived synthetically from a fermentation product of Aspergillus terreus. After oral ingestion, simvastatin, which is an inactive lactone, is hydrolyzed to the correspondingform. This is an inhibitor of 3... |
| Active Ingredient | Simvastatin |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 5mg; 10mg; 80mg; 40mg; 20mg |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharms; Ranbaxy; Accord Hlthcare; Aurobindo Pharma; Lupin; Prosam Labs; Watson Labs; Blu Caribe; Ivax Sub Teva Pharms; Teva Pharms; Zydus Pharms Usa; Dr Reddys Labs; Micro Labs |
| 2 of 4 | |
|---|---|
| Drug Name | Zocor |
| PubMed Health | Simvastatin (By mouth) |
| Drug Classes | Antihyperlipidemic |
| Drug Label | ZOCOR (simvastatin) is a lipid-lowering agent that is derived synthetically from a fermentation product of Aspergillus terreus. After oral ingestion, simvastatin, which is an inactive lactone, is hydrolyzed to the corresponding -hydroxyacid form. T... |
| Active Ingredient | Simvastatin |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 10mg; 80mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Merck |
| 3 of 4 | |
|---|---|
| Drug Name | Simvastatin |
| PubMed Health | Simvastatin (By mouth) |
| Drug Classes | Antihyperlipidemic |
| Drug Label | Simvastatin is a lipid-lowering agent that is derived synthetically from a fermentation product of Aspergillus terreus. After oral ingestion, simvastatin, which is an inactive lactone, is hydrolyzed to the correspondingform. This is an inhibitor of 3... |
| Active Ingredient | Simvastatin |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 5mg; 10mg; 80mg; 40mg; 20mg |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharms; Ranbaxy; Accord Hlthcare; Aurobindo Pharma; Lupin; Prosam Labs; Watson Labs; Blu Caribe; Ivax Sub Teva Pharms; Teva Pharms; Zydus Pharms Usa; Dr Reddys Labs; Micro Labs |
| 4 of 4 | |
|---|---|
| Drug Name | Zocor |
| PubMed Health | Simvastatin (By mouth) |
| Drug Classes | Antihyperlipidemic |
| Drug Label | ZOCOR (simvastatin) is a lipid-lowering agent that is derived synthetically from a fermentation product of Aspergillus terreus. After oral ingestion, simvastatin, which is an inactive lactone, is hydrolyzed to the corresponding -hydroxyacid form. T... |
| Active Ingredient | Simvastatin |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 10mg; 80mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Merck |
Anticholesteremic Agents; Hydroxymethylglutaryl-CoA Reductase Inhibitors
National Library of Medicine's Medical Subject Headings. Simvastatin. Online file (MeSH, 2016). Available from, as of November 28, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Simvastatin is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of February 1, 2017: https://clinicaltrials.gov/ct2/results?term=SIMVASTATIN&Search=Search
Zocor is indicated as an adjunct to diet to reduce total cholesterol (total-C), low-density lipoprotein cholesterol (LDL-C), and Apo B levels in adolescent boys and girls who are at least one year post-menarche, 10-17 years of age, with HeFH, if after an adequate trial of diet therapy the following findings are present: LDL cholesterol remains >/= 190 mg/dL; or LDL cholesterol remains >/= 160 mg/dL and there is a positive family history of premature cardiovascular disease (CVD) or two or more other CVD risk factors are present in the adolescent patient. The minimum goal of treatment in pediatric and adolescent patients is to achieve a mean LDL-C <130 mg/dL. The optimal age at which to initiate lipid-lowering therapy to decrease the risk of symptomatic adulthood CAD has not been determined. /Included in US product label/
NIH; DailyMed. Current Medication Information for Zocor (Simvastatin Tablet, Film-Coated) (Updated: March 2015). Available from, as of February 3, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fdbfe194-b845-42c5-bb87-a48118bc72e7
Zocor is indicated to: Reduce elevated total cholesterol (total-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein B (Apo B), and triglycerides (TG), and to increase high-density lipoprotein cholesterol (HDL-C) in patients with primary hyperlipidemia (Fredrickson type IIa, heterozygous familial and nonfamilial) or mixed dyslipidemia (Fredrickson type IIb); Reduce elevated TG in patients with hypertriglyceridemia (Fredrickson type lV hyperlipidemia); Reduce elevated TG and VLDL-C in patients with primary dysbetalipoproteinemia (Fredrickson type III hyperlipidemia); Reduce total-C and LDL-C in patients with homozygous familial hypercholesterolemia (HoFH) as an adjunct to other lipid-lowering treatments (e.g., LDL apheresis) or if such treatments are unavailable.
NIH; DailyMed. Current Medication Information for Zocor (Simvastatin Tablet, Film-Coated) (Updated: March 2015). Available from, as of February 3, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fdbfe194-b845-42c5-bb87-a48118bc72e7
For more Therapeutic Uses (Complete) data for Simvastatin (11 total), please visit the HSDB record page.
Zocor is contraindicated in women who are or may become pregnant. Lipid lowering drugs offer no benefit during pregnancy, because cholesterol and cholesterol derivatives are needed for normal fetal development. Atherosclerosis is a chronic process, and discontinuation of lipid-lowering drugs during pregnancy should have little impact on long-term outcomes of primary hypercholesterolemia therapy. ... Serum cholesterol and triglycerides increase during normal pregnancy, and cholesterol or cholesterol derivatives are essential for fetal development. Because statins decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol, Zocor may cause fetal harm when administered to a pregnant woman. If Zocor is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
NIH; DailyMed. Current Medication Information for Zocor (Simvastatin Tablet, Film-Coated) (Updated: March 2015). Available from, as of February 3, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fdbfe194-b845-42c5-bb87-a48118bc72e7
Grapefruit juice contains one or more components that inhibit CYP3A4 and can increase the plasma levels of drugs metabolized by CYP3A4. The effect of typical consumption (one 250-ml glass daily) is minimal (13% increase in active plasma HMG-CoA reductase inhibitory activity as measured by the area under the concentration-time curve) and of no clinical relevance. However, because larger quantities significantly increase the plasma levels of HMG-CoA reductase inhibitory activity, grapefruit juice should be avoided during simvastatin therapy.
Health Canada; Product Monograph for Simvastatin Tablets USP (5 mg,10 mg, 20 mg,40 mg and 80 mg), Drug Identification Number (DIN): 02374625 p.17 (Date of Revision: January 4, 2016). Available from, as of February 3, 2017: https://health-products.canada.ca/dpd-bdpp/index-eng.jsp
It is not known whether simvastatin is excreted in human milk. Because a small amount of another drug in this class is excreted in human milk and because of the potential for serious adverse reactions in nursing infants, women taking simvastatin should not nurse their infants. A decision should be made whether to discontinue nursing or discontinue drug, taking into account the importance of the drug to the mother.
NIH; DailyMed. Current Medication Information for Zocor (Simvastatin Tablet, Film-Coated) (Updated: March 2015). Available from, as of February 3, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fdbfe194-b845-42c5-bb87-a48118bc72e7
Because advanced age (>/= 65 years) is a predisposing factor for myopathy, including rhabdomyolysis, Zocor should be prescribed with caution in the elderly. In a clinical trial of patients treated with simvastatin 80 mg/day, patients >/= 65 years of age had an increased risk of myopathy, including rhabdomyolysis, compared to patients <65 years of age.
NIH; DailyMed. Current Medication Information for Zocor (Simvastatin Tablet, Film-Coated) (Updated: March 2015). Available from, as of February 3, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fdbfe194-b845-42c5-bb87-a48118bc72e7
For more Drug Warnings (Complete) data for Simvastatin (33 total), please visit the HSDB record page.
Simvastatin is indicated for the treatment of hyperlipidemia to reduce elevated total cholesterol (total-C), low-density lipoprotein cholesterol (LDLC), apolipoprotein B (Apo B), and triglycerides (TG), and to increase high-density lipoprotein cholesterol (HDL-C). This includes the treatment of primary hyperlipidemia (Fredrickson type IIa, heterozygous familial and nonfamilial), mixed dyslipidemia (Fredrickson type IIb), hypertriglyceridemia (Fredrickson type IV hyperlipidemia), primary dysbetalipoproteinemia (Fredrickson type III hyperlipidemia), homozygous familial hypercholesterolemia (HoFH) as an adjunct to other lipid-lowering treatments, as well as adolescent patients with Heterozygous Familial Hypercholesterolemia (HeFH). Simvastatin is also indicated to reduce the risk of cardiovascular morbidity and mortality including myocardial infarction, stroke, and the need for revascularization procedures. It is primarily used in patients at high risk of coronary events because of existing coronary heart disease, diabetes, peripheral vessel disease, history of stroke or other cerebrovascular disease. Prescribing of statin medications is considered standard practice following any cardiovascular events and for people with a moderate to high risk of development of CVD. Statin-indicated conditions include diabetes mellitus, clinical atherosclerosis (including myocardial infarction, acute coronary syndromes, stable angina, documented coronary artery disease, stroke, trans ischemic attack (TIA), documented carotid disease, peripheral artery disease, and claudication), abdominal aortic aneurysm, chronic kidney disease, and severely elevated LDL-C levels.
Simvastatin is an oral antilipemic agent which inhibits HMG-CoA reductase. It is used to lower total cholesterol, low density lipoprotein-cholesterol (LDL-C), apolipoprotein B (apoB), non-high density lipoprotein-cholesterol (non-HDL-C), and trigleride (TG) plasma concentrations while increasing HDL-C concentrations. High LDL-C, low HDL-C and high TG concentrations in the plasma are associated with increased risk of atherosclerosis and cardiovascular disease. The total cholesterol to HDL-C ratio is a strong predictor of coronary artery disease and high ratios are associated with higher risk of disease. Increased levels of HDL-C are associated with lower cardiovascular risk. By decreasing LDL-C and TG and increasing HDL-C, rosuvastatin reduces the risk of cardiovascular morbidity and mortality. Elevated cholesterol levels, and in particular, elevated low-density lipoprotein (LDL) levels, are an important risk factor for the development of CVD. Use of statins to target and reduce LDL levels has been shown in a number of landmark studies to significantly reduce the risk of development of CVD and all-cause mortality. Statins are considered a cost-effective treatment option for CVD due to their evidence of reducing all-cause mortality including fatal and non-fatal CVD as well as the need for surgical revascularization or angioplasty following a heart attack. Evidence has shown that even for low-risk individuals (with <10% risk of a major vascular event occurring within 5 years) statins cause a 20%-22% relative reduction in major cardiovascular events (heart attack, stroke, coronary revascularization, and coronary death) for every 1 mmol/L reduction in LDL without any significant side effects or risks. **Skeletal Muscle Effects** Simvastatin occasionally causes myopathy manifested as muscle pain, tenderness or weakness with creatine kinase (CK) above ten times the upper limit of normal (ULN). Myopathy sometimes takes the form of rhabdomyolysis with or without acute renal failure secondary to myoglobinuria, and rare fatalities have occurred. Predisposing factors for myopathy include advanced age (65 years), female gender, uncontrolled hypothyroidism, and renal impairment. Chinese patients may also be at increased risk for myopathy. In most cases, muscle symptoms and CK increases resolved when treatment was promptly discontinued. In a clinical trial database of 41,413 patients, the incidence of myopathy was approximately 0.03% and 0.08% at 20 and 40 mg/day, respectively, while the risk of myopathy with simvastatin 80 mg (0.61%) was disproportionately higher than that observed at the lower doses. It's therefore recommended that the 80mg dose of simvastatin should be used only in patients who have been taking simvastatin 80 mg chronically (e.g., for 12 months or more) without evidence of muscle toxicity. As well, patients already stabilized on simvastatin 80mg should be monitored closely for evidence of muscle toxicity; if they need to be initiated on an interacting drug that is contraindicated or is associated with a dose cap for simvastatin, that patient should be switched to an alternative statin with less potential for the drug-drug interaction. The risk of myopathy during treatment with simvastatin may be increased with concurrent administration of interacting drugs such as [fenofibrate], [niacin], [gemfibrozil], [cyclosporine], and strong inhibitors of the CYP3A4 enzyme. Cases of myopathy, including rhabdomyolysis, have been reported with HMG-CoA reductase inhibitors coadministered with [colchicine], and caution should therefore be exercised when prescribing these two medications together. **Liver Enzyme Abnormalities** Persistent increases (to more than 3X the ULN) in serum transaminases have occurred in approximately 1% of patients who received simvastatin in clinical studies. When drug treatment was interrupted or discontinued in these patients, the transaminase levels usually fell slowly to pretreatment levels. The increases were not associated with jaundice or other clinical signs or symptoms. In the Scandinavian Simvastatin Survival Study (4S), the number of patients with more than one transaminase elevation to >3 times the ULN, over the course of the study, was not significantly different between the simvastatin and placebo groups (14 [0.7%] vs. 12 [0.6%]). The frequency of single elevations of ALT to 3 times the ULN was significantly higher in the simvastatin group in the first year of the study (20 vs. 8, p=0.023), but not thereafter. In the HPS (Heart Protection Study), in which 20,536 patients were randomized to receive simvastatin 40 mg/day or placebo, the incidences of elevated transaminases (>3X ULN confirmed by repeat test) were 0.21% (n=21) for patients treated with simvastatin and 0.09% (n=9) for patients treated with placebo. **Endocrine Effects** Increases in HbA1c and fasting serum glucose levels have been reported with HMG-CoA reductase inhibitors, including simvastatin. Although cholesterol is the precursor of all steroid hormones, studies with simvastatin have suggested that this agent has no clinical effect on steroidogenesis. Simvastatin caused no increase in biliary lithogenicity and, therefore, would not be expected to increase the incidence of gallstones.
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Compounds that inhibit HYDROXYMETHYLGLUTARYL COA REDUCTASES. They have been shown to directly lower CHOLESTEROL synthesis. (See all compounds classified as Hydroxymethylglutaryl-CoA Reductase Inhibitors.)
Hypolipidemic Agents
Substances that lower the levels of certain LIPIDS in the BLOOD. They are used to treat HYPERLIPIDEMIAS. (See all compounds classified as Hypolipidemic Agents.)
Anticholesteremic Agents
Substances used to lower plasma cholesterol levels. (See all compounds classified as Anticholesteremic Agents.)
C10AA01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C10 - Lipid modifying agents
C10A - Lipid modifying agents, plain
C10AA - Hmg coa reductase inhibitors
C10AA01 - Simvastatin
Absorption
Peak plasma concentrations of both active and total inhibitors were attained within 1.3 to 2.4 hours post-dose. While the recommended therapeutic dose range is 10 to 40 mg/day, there was no substantial deviation from linearity of AUC with an increase in dose to as high as 120 mg. Relative to the fasting state, the plasma profile of inhibitors was not affected when simvastatin was administered immediately before a test meal. In a pharmacokinetic study of 17 healthy Chinese volunteers, the major PK parameters were as follows: Tmax 1.44 hours, Cmax 9.83 ug/L, t1/2 4.85 hours, and AUC 40.32ugh/L. Simvastatin undergoes extensive first-pass extraction in the liver, the target organ for the inhibition of HMG-CoA reductase and the primary site of action. This tissue selectivity (and consequent low systemic exposure) of orally administered simvastatin has been shown to be far greater than that observed when the drug is administered as the enzymatically active form, i.e. as the open hydroxyacid. In animal studies, after oral dosing, simvastatin achieved substantially higher concentrations in the liver than in non-target tissues. However, because simvastatin undergoes extensive first-pass metabolism, the bioavailability of the drug in the systemic system is low. In a single-dose study in nine healthy subjects, it was estimated that less than 5% of an oral dose of simvastatin reached the general circulation in the form of active inhibitors. Genetic differences in the OATP1B1 (Organic-Anion-Transporting Polypeptide 1B1) hepatic transporter encoded by the SCLCO1B1 gene (Solute Carrier Organic Anion Transporter family member 1B1) have been shown to impact simvastatin pharmacokinetics. Evidence from pharmacogenetic studies of the c.521T>C single nucleotide polymorphism (SNP) showed that simvastatin plasma concentrations were increased on average 3.2-fold for individuals homozygous for 521CC compared to homozygous 521TT individuals. The 521CC genotype is also associated with a marked increase in the risk of developing myopathy, likely secondary to increased systemic exposure. Other statin drugs impacted by this polymorphism include [rosuvastatin], [pitavastatin], [atorvastatin], [lovastatin], and [pravastatin]. For patients known to have the above-mentioned c.521CC OATP1B1 genotype, a maximum daily dose of 20mg of simvastatin is recommended to avoid adverse effects from the increased exposure to the drug, such as muscle pain and risk of rhabdomyolysis. Evidence has also been obtained with other statins such as [rosuvastatin] that concurrent use of statins and inhibitors of Breast Cancer Resistance Protein (BCRP) such as elbasvir and grazoprevir increased the plasma concentration of these statins. Further evidence is needed, however a dose adjustment of simvastatin may be necessary. Other statin drugs impacted by this polymorphism include [fluvastatin] and [atorvastatin].
Route of Elimination
Following an oral dose of 14C-labeled simvastatin in man, 13% of the dose was excreted in urine and 60% in feces.
Volume of Distribution
Rat studies indicate that when radiolabeled simvastatin was administered, simvastatin-derived radioactivity crossed the blood-brain barrier.
Both simvastatin and its beta-hydroxyacid metabolite are highly bound (approximately 95%) to human plasma proteins. Rat studies indicate that when radiolabeled simvastatin was administered, simvastatin-derived radioactivity crossed the blood-brain barrier.
NIH; DailyMed. Current Medication Information for Zocor (Simvastatin Tablet, Film-Coated) (Updated: March 2015). Available from, as of February 3, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fdbfe194-b845-42c5-bb87-a48118bc72e7
/MILK/ It is not known whether simvastatin is distributed into human breast milk ... .
NIH; DailyMed. Current Medication Information for Zocor (Simvastatin Tablet, Film-Coated) (Updated: March 2015). Available from, as of February 3, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fdbfe194-b845-42c5-bb87-a48118bc72e7
Following an oral dose of (14)C-labeled simvastatin in man, 13% of the dose was excreted in urine and 60% in feces. Plasma concentrations of total radioactivity (simvastatin plus (14)C-metabolites) peaked at 4 hours and declined rapidly to about 10% of peak by 12 hours postdose. Since simvastatin undergoes extensive first-pass extraction in the liver, the availability of the drug to the general circulation is low (<5%).
NIH; DailyMed. Current Medication Information for Zocor (Simvastatin Tablet, Film-Coated) (Updated: March 2015). Available from, as of February 3, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fdbfe194-b845-42c5-bb87-a48118bc72e7
Absorption, distribution and excretion of (14)C-simvastatin were studied in male rats after 21-day consecutive daily oral administration at the dose of 10 mg/kg. Plasma levels of (14C)simvastatin at 1hr after each administration did not increase during and after repeated administration. The radioactivity levels-time curve after the final administration was similar to that after the first dosing. The cumulative excretion of radioactivity in urine and feces accounted for 9.0% and 91.4% of the total dose, respectively, within 96hr after the final administration. After the final administration, radioactivity was concentrated in the gastrointestinal tracts, liver and kidney. The distribution pattern was similar to that observed after the single administration. There was no accumulation of the drug and its metabolites in the tissues of rats after the consecutive oral administration of (14)C-simvastatin. Foeto-placental transfer and excretion of radioactivity into milk were studied in pregnant and lactating rats after single oral administration of (14)C-simvastatin. Whole body autoradiograms of rats on day 12 and 18 of gestation showed low distribution and rapid elimination of radioactivity from the fetus. On day 18 of gestation, the concentration of radioactivity in the placenta, amniotic fluid and fetal tissues were nearly equal to or less than those in the maternal plasma. The amount of radioactivity transferred into a fetus was about 0.02% of the oral dose. The concentrations of radioactivity in the milk were about 20-54% of those in maternal plasma.
Ohtawa M et al; Yakubutsu Dotai 5 (2): 21-33 (1990)
For more Absorption, Distribution and Excretion (Complete) data for Simvastatin (6 total), please visit the HSDB record page.
Simvastatin is administered as the inactive lactone derivative that is then metabolically activated to its -hydroxyacid form by a combination of spontaneous chemical conversion and enzyme-mediated hydrolysis by nonspecific carboxyesterases in the intestinal wall, liver, and plasma. Oxidative metabolism in the liver is primarily mediated by CYP3A4 and CYP3A5, with the remaining metabolism occurring through CYP2C8 and CYP2C9. The major active metabolites of simvastatin are -hydroxyacid metabolite and its 6'-hydroxy, 6'-hydroxymethyl, and 6'-exomethylene derivatives. Polymorphisms in the CYP3A5 gene have been shown to affect the disposition of simvastatin and may provide a plausible explanation for interindividual variability of simvastatin disposition and pharmacokinetics.
The major active metabolites of simvastatin present in human plasma are the beta-hydroxyacid of simvastatin and its 6'-hydroxy, 6'-hydroxymethyl, and 6'-exomethylene derivatives.
NIH; DailyMed. Current Medication Information for Zocor (Simvastatin Tablet, Film-Coated) (Updated: March 2015). Available from, as of February 3, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fdbfe194-b845-42c5-bb87-a48118bc72e7
Simvastatin has known human metabolites that include 3', 5'-Dihydrodiol, 6'-alpha-Hydroxysimvastatin, and 6'-exomethylene.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
4.85 hours
Simvastatin is a prodrug in which the 6-membered lactone ring of simvastatin is hydrolyzed in vivo to generate the beta,delta-dihydroxy acid, an active metabolite structurally similar to HMG-CoA (hydroxymethylglutaryl CoA). Once hydrolyzed, simvastatin competes with HMG-CoA for HMG-CoA reductase, a hepatic microsomal enzyme, which catalyzes the conversion of HMG-CoA to mevalonate, an early rate-limiting step in cholesterol biosynthesis. Simvastatin acts primarily in the liver, where decreased hepatic cholesterol concentrations stimulate the upregulation of hepatic low density lipoprotein (LDL) receptors which increases hepatic uptake of LDL. Simvastatin also inhibits hepatic synthesis of very low density lipoprotein (VLDL). The overall effect is a decrease in plasma LDL and VLDL. At therapeutic doses, the HMG-CoA enzyme is not completely blocked by simvastatin activity, thereby allowing biologically necessary amounts of mevalonate to remain available. As mevalonate is an early step in the biosynthetic pathway for cholesterol, therapy with simvastatin would also not be expected to cause any accumulation of potentially toxic sterols. In addition, HMG-CoA is metabolized readily back to acetyl-CoA, which participates in many biosynthetic processes in the body. In vitro and in vivo animal studies also demonstrate that simvastatin exerts vasculoprotective effects independent of its lipid-lowering properties, also known as the pleiotropic effects of statins. This includes improvement in endothelial function, enhanced stability of atherosclerotic plaques, reduced oxidative stress and inflammation, and inhibition of the thrombogenic response. Statins have also been found to bind allosterically to 2 integrin function-associated antigen-1 (LFA-1), which plays an important role in leukocyte trafficking and in T cell activation.
Simvastatin is a prodrug and is hydrolyzed to its active beta-hydroxyacid form, simvastatin acid, after administration. Simvastatin is a specific inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the enzyme that catalyzes the conversion of HMG-CoA to mevalonate, an early and rate limiting step in the biosynthetic pathway for cholesterol. In addition, simvastatin reduces VLDL and TG and increases HDL-C.
NIH; DailyMed. Current Medication Information for Zocor (Simvastatin Tablet, Film-Coated) (Updated: March 2015). Available from, as of February 3, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fdbfe194-b845-42c5-bb87-a48118bc72e7
The HDL-associated enzyme paraoxonase protects LDLs from oxidative stress. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) appear to favorably influence the atherosclerotic process by different mechanisms. The present study examined the influence of simvastatin on paraoxonase expression and serum paraoxonase levels. Simvastatin upregulated in a dose-dependent manner the activity of the promoter of the paraoxonase gene in expression cassettes transfected into HepG2 cells. Upregulation could be blocked by mevalonate and other intermediates of the cholesterol biosynthetic pathway. Simvastatin increased nuclear factors, notably sterol regulatory element-binding protein-2, capable of binding to the paraoxonase promoter; this was also blocked by mevalonate. Sterol regulatory element-binding protein-2 upregulated promoter activity in vitro. Patients treated with statin showed a significant increase in serum concentrations and activities of paraoxonase. The data indicate that simvastatin can modulate expression in vitro of the antioxidant enzyme paraoxonase and is associated with increased serum paraoxonase concentration and activity. It is consistent with effects of simvastatin treatment, which have the potential to influence beneficially antiatherogenic mechanisms at the HDL level. The study provides evidence for 1 molecular mechanism by which paraoxonase gene expression could be regulated.
PMID:14500290 Deakin S et al; Arterioscler Thromb Vasc Biol 23 (11): 2083-9 (2003)
... We report in this work that, unexpectedly, simvastatin enhances LPS-induced IL-12p40 production by murine macrophages, and that it does so by activating the IL-12p40 promoter. Mutational analysis and dominant-negative expression studies indicate that both C/EBP and AP-1 transcription factors have a crucial role in promoter activation. This occurs via a c-Fos- and c-Jun-based mechanism; we demonstrate that ectopic expression of c-Jun activates the IL-12p40 promoter, whereas expression of c-Fos inhibits IL-12p40 promoter activity. Simvastatin prevents LPS-induced c-Fos expression, thereby relieving the inhibitory effect of c-Fos on the IL-12p40 promoter. Concomitantly, simvastatin induces the phosphorylation of c-Jun by the c-Jun N-terminal kinase, resulting in c-Jun-dependent activation of the IL-12p40 promoter. This appears to be a general mechanism because simvastatin also augments LPS-dependent activation of the TNF-alpha promoter, perhaps because the TNF-alpha promoter has C/EBP and AP-1 binding sites in a similar configuration to the IL-12p40 promoter. The fact that simvastatin potently augments LPS-induced IL-12p40 and TNF-alpha production has implications for the treatment of bacterial infections in statin-treated patients.
PMID:15187114 Matsumoto M et al; J Immunol 172 (12): 7377-84 (2004)
Statins are increasingly recognized as mediators of direct cellular effects independent of their lipid lowering capacity. Therefore, the time and concentration dependence of various statin-mediated cellular alterations was compared in renal mesangial cells. The effects of statins on cell proliferation, gene expression, cytoskeletal alterations, apoptosis, and cytotoxicity were analyzed in cultured mesangial cells using standard techniques. Results. Simvastatin and lovastatin decreased proliferation and cell number of rat mesangial cells concentration-dependently. Concurrently, the expression of the fibrogenic protein connective tissue growth factor (CTGF) was impaired and actin stress fibers, which are typical of mesangial cells in culture, became disassembled by simvastatin. A decrease of the posttranslational modification of RhoA by geranylgeranyl moieties was detected, supporting a role for RhoA as mediator of statin effects. Induction of apoptosis, determined by activation of caspase-3 and DNA fragmentation, and necrosis only occurred at later time points, when the morphology of the cells was strongly altered and the cells detached from the surface due to changes in the actin cytoskeleton. Basically, the same results were obtained with a human mesangial cell line. Furthermore, statin effects were mimicked by inhibition of the geranylgeranyltransferase. Most of the cellular effects of the lipophilic statins occurred within the same time and concentration range, suggesting a common molecular mechanism. Only apoptosis and necrosis were observed at later time points or with higher concentrations of simvastatin and thus seem to be secondary to the changes in gene expression and alterations of the actin cytoskeleton.
PMID:15200425 Heusinger-Ribeiro J et al; Kidney Int 66 (1): 187-95 (2004)
For more Mechanism of Action (Complete) data for Simvastatin (6 total), please visit the HSDB record page.








