



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
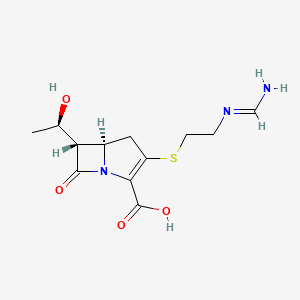

1. Anhydrous Imipenem
2. Anhydrous, Imipenem
3. Imipemide
4. Imipenem Anhydrous
5. Imipenem, Anhydrous
6. Mk 0787
7. Mk-0787
8. Mk0787
9. N Formimidoylthienamycin
10. N-formimidoylthienamycin
1. 64221-86-9
2. Imipemide
3. N-formimidoylthienamycin
4. Imipenem Anhydrous
5. Tienamycin
6. Imipenemum
7. N-formimidoyl Thienamycin
8. Imipenem Hydrate
9. Mk 0787
10. Chebi:471744
11. 74431-23-5
12. Imipenem, N-formimidoyl Thienamycin
13. (5r,6s)-3-[2-(aminomethylideneamino)ethylsulfanyl]-6-[(1r)-1-hydroxyethyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
14. Imipenem (inn)
15. Imipenem [inn]
16. (5r,6s)-6-((r)-1-hydroxyethyl)-3-(2-(iminomethylamino)ethylthio)-7-oxo-1-azabicyclo(3.2.0)hept-2-ene-2-carbonsaeure
17. (5r,6s)-3-((2-(formimidoylamino)ethyl)thio)-6-((r)-1-hydroxyethyl)-7-oxo-1-azabicyclo(3.2.0)hept-2-ene-2-carboxylic Acid
18. Mk-0787
19. Chembl148
20. Q20im7he75
21. (5r,6s)-3-(2-formimidoylamino-ethylsulfanyl)-6-((r)-1-hydroxy-ethyl)-7-oxo-1-aza-bicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
22. Imipenen
23. Dsstox_cid_3143
24. Dsstox_rid_76888
25. Dsstox_gsid_23143
26. Anhydrous Imipenem
27. Imipenem, Anhydrous
28. Imipenemum [latin]
29. Cas-64221-86-9
30. Sr-05000000294
31. Imipen
32. Unii-q20im7he75
33. Ncgc00016928-01
34. (5r,6s)-6-[(1r)-1-hydroxyethyl]-3-(2-methanimidamidoethylsulfanyl)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
35. 1-azabicyclo(3.2.0)hept-2-ene-2-carboxylic Acid, 6-((1r)-1-hydroxyethyl)-3-((2-((iminomethyl)amino)ethyl)thio)-7-oxo-, (5r,6s)-
36. 1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid, 6-[(1r)-1-hydroxyethyl]-3-[[2-[(iminomethyl)amino]ethyl]thio]-7-oxo-, (5r,6s)-
37. Prestwick_844
38. Einecs 264-734-5
39. Mk-787
40. Mk0787
41. Imipenem [mi]
42. Imipenem Anhydrate
43. Prestwick0_000519
44. Prestwick1_000519
45. Prestwick2_000519
46. Prestwick3_000519
47. Epitope Id:120384
48. Imipenem [who-dd]
49. Bspbio_000477
50. Bidd:gt0686
51. Spbio_002398
52. Bpbio1_000525
53. Schembl1649260
54. Schembl8781920
55. Dtxsid2023143
56. Gtpl10821
57. Hy-b1369a
58. Primaxin (imipenem + Cilastatin)
59. Hms1569h19
60. Hms2090a15
61. Hms2096h19
62. Hms3260h20
63. Hms3713h19
64. Pharmakon1600-01506001
65. Bcp13012
66. Zinc4097225
67. Tox21_110689
68. Tox21_500279
69. Bdbm50049708
70. Bdbm50213266
71. Nsc717864
72. Nsc759901
73. Akos016010844
74. Tox21_110689_1
75. Ccg-220519
76. Ccg-221583
77. Db01598
78. Lp00279
79. Nsc-717864
80. Sdccgsbi-0633697.p001
81. Ncgc00167958-01
82. Ncgc00167958-02
83. Ncgc00167958-03
84. Ncgc00167958-05
85. Ncgc00167958-09
86. Ncgc00260964-01
87. (5r,6s)-6-[(1r)-1-hydroxyethyl]-3-({2-[(iminomethyl)amino]ethyl}thio)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
88. 1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid,6-[(1r)-1-hydroxyethyl]-3-[[2-[(iminomethyl)amino]ethyl]thio]-7-oxo-,(5r,6s)-
89. As-75130
90. Cs-0077844
91. C06665
92. D04515
93. D96091
94. Ab01563339_01
95. Recarbrio (imipenem + Cilastatin + Relebactam).
96. 847i667
97. Q425152
98. Sr-05000000294-2
99. Sr-05000000294-5
100. Thienamycin P-nitrobenzylester Hydrochloride(n-methylpyrrolidinonesolvate)
101. (5r,6s)-3-((2-formimidamidoethyl)thio)-6-((r)-1-hydroxyethyl)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
102. (5r,6s)-3-({2-[(e)-(aminomethylidene)amino]ethyl}sulfanyl)-6-[(1r)-1-hydroxyethyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
103. (5r,6s)-3-(2-formimidamidoethylthio)-6-((r)-1-hydroxyethyl)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
104. (5r,6s)-6-[(1r)-1-hydroxyethyl]-3-[(2-methanimidamidoethyl)sulfanyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
105. [5r-[5.alpha.,6.alpha.(r*)]]-6-(1-hydroxyethyl)-3-[[2- [(iminomethyl)amino]ethyl]thio]-7-oxo-1-azabicyclo[3.2.0]hept-2- Ene-2-carboxylic Acid Monohydrate
106. 1-azabicyclo(3.2.0)hept-2-ene-2-carboxylic Acid, 6-(1-hydroxyethyl)-3-((2-((iminomethyl)amino)ethyl)thio)-7-oxo-, (5r-(5-alpha,6-alpha(r*)))-
107. 1-azabicyclo(3.2.0)hept-2-ene-2-carboxylic Acid, 6-(1-hydroxyethyl)-3-((2-((iminomethyl)amino)ethyl)thio)-7-oxo-, (5r-(5.alpha.,6.alpha.(r*)))-
108. 103730-39-8
109. 3-[(2-aminoethyl)thio]-6-[(1r)-1-hydroxyethyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid (4-nitrophenyl)methylester Monohydrochloride Compd. With 1-methyl-2-pyrrolidinone (1:1)
| Molecular Weight | 299.35 g/mol |
|---|---|
| Molecular Formula | C12H17N3O4S |
| XLogP3 | -0.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 299.09397721 g/mol |
| Monoisotopic Mass | 299.09397721 g/mol |
| Topological Polar Surface Area | 142 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 491 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Primaxin |
| PubMed Health | Imipenem/Cilastatin (Injection) |
| Drug Classes | Antibiotic |
| Active Ingredient | imipenem; Cilastatin sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 500mg/vial; 250mg/vial; eq 500mg base/vial; eq 250mg base/vial |
| Market Status | Prescription |
| Company | Merck |
| 2 of 2 | |
|---|---|
| Drug Name | Primaxin |
| PubMed Health | Imipenem/Cilastatin (Injection) |
| Drug Classes | Antibiotic |
| Active Ingredient | imipenem; Cilastatin sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 500mg/vial; 250mg/vial; eq 500mg base/vial; eq 250mg base/vial |
| Market Status | Prescription |
| Company | Merck |
Imipenem is indicated, in combination with [cilastatin] with or without [relebactam], for the treatment of bacterial infections including respiratory, skin, bone, gynecologic, urinary tract, and intra-abdominal as well as septicemia and endocarditis.
FDA Label
Imipenem is a beta-lactam antibiotic belongings to the subgroup of carbapenems. Imipenem is active against aerobic and anaerobic Gram positive as well as Gram negative bacteria including Pseudomonas aeruginosa and the Enterococcus. It exerts a bactericidal effects by disrupting cell wall synthesis.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01DH51
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
Absorption
Imipenem is not effectively absorbed from the gastrointestinal tract and therefore must be administered parenterally. The bioavailability of the IM injection is 89%.
Route of Elimination
Approximately 70% of imipenem is excreted in the urine as the parent drug.
Volume of Distribution
The reported volume of distribution for imipenem ranges from 0.23-0.31 L/kg.
Clearance
The total clearance of imipenem is 0.2 L/h/kg. When used alone, the renal clearance is 0.05 L/h/kg. In combination with cilastatin the renal clearance of imipenem is 0.15 L/h/kg, likely due to the increased concentration of the parent drug.
Imipenem is metabolized by renal dehydropeptidase.
When given via IV injection imipenem has a half-life of 1 h. The apparent half-life of the IM injection ranges from 1.3-5.1 h, likely due to slower absorption form the injection site.
Imipenem acts as an antimicrobial through the inhibition of cell wall synthesis of various gram-positive and gram-negative bacteria. This inhibition of cell wall synthesis in gram-negative bateria is attained by binding to penicillin-binding proteins (PBPs). In E. coli and selected strains of P. aeruginosa, imipenem has shown to have the highest affinity to PBP-2, PBP-1a, and PBP-1b. This inhibition of PBPs prevents the bacterial cell from adding to the peptidoglycan polymer which forms the bacterial cell wall eventually leading to cell death.




