
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz

1. Ammonium Salt Ditiocarb
2. Bismuth Salt Ditiocarb
3. Diethylcarbamodithioic Acid
4. Diethyldithiocarbamate
5. Diethyldithiocarbamate, Sodium
6. Diethyldithiocarbamate, Zinc
7. Diethyldithiocarbamic Acid
8. Dithiocarb
9. Ditiocarb
10. Ditiocarb Sodium
11. Ditiocarb, Ammonium Salt
12. Ditiocarb, Bismuth Salt
13. Ditiocarb, Lead Salt
14. Ditiocarb, Potassium Salt
15. Ditiocarb, Sodium Salt
16. Ditiocarb, Sodium Salt, Trihydrate
17. Ditiocarb, Tin(4+) Salt
18. Ditiocarb, Zinc Salt
19. Imuthiol
20. Lead Salt Ditiocarb
21. Potassium Salt Ditiocarb
22. Sodium Salt Ditiocarb
23. Sodium, Ditiocarb
24. Thiocarb
25. Zinc Diethyldithiocarbamate
26. Zinc Salt Ditiocarb
1. 148-18-5
2. Ditiocarb Sodium
3. Sodium N,n-diethyldithiocarbamate
4. Dithiocarb
5. Thiocarb
6. Imuthiol
7. Dedtc
8. Sodium Diethylcarbamodithioate
9. Dedc
10. Ditiocarb Sodium [inn]
11. Ddtc
12. Carbamodithioic Acid, Diethyl-, Sodium Salt
13. Diethyldithiocarbamate Sodium
14. Cupral
15. Diethyldithiocarbamic Acid, Sodium Salt
16. Sodium Dedt
17. N,n-diethyldithiocarbamic Acid, Sodium Salt
18. A5304yeb5e
19. Sodium N,n-diethyldithiocarbamate (25% Solution In Water)
20. Usaf Ek-2596
21. Chebi:82587
22. Diethyldithiocarbamic Acid Sodium Salt
23. Diethyl Sodium Dithiocarbamate
24. Nsc-38583
25. Nci Co2835
26. Carbamodithioic Acid, N,n-diethyl-, Sodium Salt (1:1)
27. Sodium Diethylaminocarbodithioate
28. Diethyldithiocarbamic Acid, Sodium
29. Gs 694a
30. Diethyl Dithiocarbamate Sodium Salt
31. Dsstox_cid_2956
32. Dsstox_rid_76806
33. Diethylcarbamodithioic Acid, Sodium Salt
34. Dsstox_gsid_22956
35. N,n-diethyl(sodiosulfanyl)carbothioamide
36. Sodium Salt Of N,n-diethyldithiocarbamic Acid
37. Kupral
38. Dtc
39. Ditiocarbo Sodico
40. Na-ddtc
41. Nocceler Sdc
42. Chembl107217
43. Soxinol Esl
44. Ditiocarbe Sodique
45. Nsc4857
46. Cas-148-18-5
47. Ditiocarbum Natricum
48. Ncgc00166328-01
49. Ditiocarbe Sodique [french]
50. Ditiocarbo Sodico [spanish]
51. Unii-a5304yeb5e
52. Ditiocarbum Natricum [latin]
53. Ccris 235
54. Hsdb 4091
55. Einecs 205-710-6
56. Nsc 38583
57. Sodium (diethylcarbamothioyl)sulfanide
58. Ai3-14688
59. Carbamic Acid, Diethyldithio-, Sodium Salt
60. Ec 205-710-6
61. Sodiumdiethylcarbamodithioate
62. Ditiocarb Sodium [mi]
63. Schembl168133
64. Ditiocarb Sodium [hsdb]
65. Dtxsid3022956
66. Ditiocarb Sodium [mart.]
67. Ditiocarb Sodium [who-dd]
68. Sodium;n,n-diethylcarbamodithioate
69. Hy-b1637
70. Tox21_112412
71. Tox21_200403
72. Mfcd00066667
73. Akos015897389
74. Akos016358586
75. Akos025310122
76. Ncgc00257957-01
77. As-12092
78. Sodium Diethyldithiocarbamate [iarc]
79. Cs-0013580
80. Ft-0631848
81. C19599
82. D78053
83. Q413008
84. J-008449
85. F8880-1809
86. Z1522566622
| Molecular Weight | 171.3 g/mol |
|---|---|
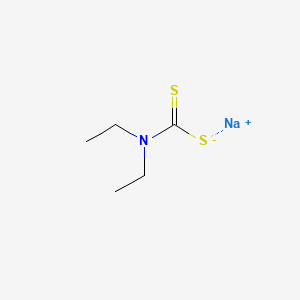
| Molecular Formula | C5H10NNaS2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 171.01523595 g/mol |
| Monoisotopic Mass | 171.01523595 g/mol |
| Topological Polar Surface Area | 36.3 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 83 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Adjuvants, Immunologic; Antiviral Agents; Chelating Agents
National Library of Medicine's Medical Subject Headings. Ditiocarb. Online file (MeSH, 2016). Available from, as of August 12, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Sodium Diethyldithiocarbamate is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of March 17, 2016: https://clinicaltrials.gov/ct2/results?term=SODIUM+DIETHYLDITHIOCARBAMATE&Search=Search
/EXPL THER/ A brief historical resume is presented on the use of dithiocarbamates for the treatment of persons exposed to nickel carbonyl. The specificity of the treatment is demonstrated in an industrial accident in which four men were simultaneously exposed to nickel carbonyl vapors. Three of the men received dithiocarb within 24 hours after exposure and the fourth was hospitalized by his family physician and treated for bronchopneumonia with antibiotics and without benefit of dithiocarb. The three workmen who received dithiocarb became symptomless and returned to work within 72 hours after exposure. The fourth man who had not received dithiocarb died within five days after exposure.
PMID:217297 Sunderman FW; Ann Clin Lab Sci. 9 (1): 1-10 (1979)
/EXPL THER/ We randomized 389 symptomatic patients with human immunodeficiency virus (HIV) infection to ditiocarb sodium (400 mg/cu m orally for 24 weeks) or a placebo. Patients were well balanced according to Centers for Disease Control (CDC) group, CD4+ cell number, and duration of disease prior to entry. Ten new acquired immunodeficiency syndrome (AIDS)-defining opportunistic infections occurred in the treated patients and 21 in the controls. Reduction of new opportunistic infections in the ditiocarb group was significant in all patients (relative risk [RR], 0.44) and in patients with AIDS (CDC groups IV-C1 and IV-D) (RR, 0.12). The size of the effect of ditiocarb was maintained when data were reanalyzed after exclusion of a patient who progressed to Pneumocystis carinii pneumonia who was not strictly CDC-defined (RR, 0.46), or when considering as new opportunistic infections three events, which were clinically active at entry, but for which the definitive diagnosis was made during study (RR, 0.49). The administration of ditiocarb did not induce any major adverse clinical or biological reactions. We conclude that, in this study, ditiocarb was safe and reduced the incidence of opportunistic infections in patients with symptomatic HIV infection.
PMID:1671884 Hersh EM et al; JAMA. 265 (12): 1538-44 (1991)
For more Therapeutic Uses (Complete) data for SODIUM DIETHYLDITHIOCARBAMATE (10 total), please visit the HSDB record page.
Chelating Agents
Chemicals that bind to and remove ions from solutions. Many chelating agents function through the formation of COORDINATION COMPLEXES with METALS. (See all compounds classified as Chelating Agents.)
Adjuvants, Immunologic
Substances that augment, stimulate, activate, potentiate, or modulate the immune response at either the cellular or humoral level. The classical agents (Freund's adjuvant, BCG, Corynebacterium parvum, et al.) contain bacterial antigens. Some are endogenous (e.g., histamine, interferon, transfer factor, tuftsin, interleukin-1). Their mode of action is either non-specific, resulting in increased immune responsiveness to a wide variety of antigens, or antigen-specific, i.e., affecting a restricted type of immune response to a narrow group of antigens. The therapeutic efficacy of many biological response modifiers is related to their antigen-specific immunoadjuvanticity. (See all compounds classified as Adjuvants, Immunologic.)
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
Rats receiving levels of 500 mg of dithiocarb in 1-2 mL water/kg body weight by gavage reached plasma levels of 2 mg/L dithiocarb in 3 hours.
Baselt RC, Hanson VW; Res Commun Chem Patbol Pharmacol 38 (1): 113-24 (1982) as cited in USEPA; Health and Environmental Effects Profile for Sodium Diethyldithiocarbamate p.7 (1983) ECAO-CIN-016
A half life for absorption of 26 minutes was determined when S-dithiocarb dissolved in 2M phosphate buffer was injected into the small intestinal lumen of adult male Wistar rats at a dose of 25 mg/kg.
Craven MR et al; J Pharm Pharmacol 28 (Suppl): 38 (1976) as cited in USEPA; Health and Environmental Effects Profile for Sodium Diethyldithiocarbamate p.7 (1983) ECAO-016
Within 15 minutes of dosing rats with 25 mg (222 moles S)/rat S-dithiocarb i.p., nonprotein bound radiolabel was detected in the plasma (1561 nmoles/mL plasma) and in the liver (3211 nmoles/g liver). A substantial amount (>45% within 15 minutes of dosing) of radioactivity also was found to be bound reversibly to soluble proteins of liver and plasma.
Stromme JH; Biochem Pharmacol 14: 393-410 (1965) as cited in USEPA; Health and Environmental Effects Profile for Sodium Diethyldithiocarbamate p.7 (1983) ECAO-CIN-016
A small amount (<0.1%) of unchanged dithiocarb was detected in the urine of rats receiving ip injections of 25 mg (35)S-dithiocarb/rat. One hr after dosing, 96.1% of the radiolabeled urinary metabolites was of S-glucuronide conjugate and 3.9% inorganic sulfate. Within 1 hr after dosing, 7% of the administered (35)S-dithiocarb was recovered as carbon disulfide in the expired air.
Stromme JH; Biochem Pharmacol 14: 393-410 (1965) as cited in USEPA; Health and Environmental Effects Profile for Sodium Diethyldithiocarbamate p.7 (1983) ECAO-CIN-016
For more Absorption, Distribution and Excretion (Complete) data for SODIUM DIETHYLDITHIOCARBAMATE (7 total), please visit the HSDB record page.
In rats, four metabolites, diethyldithiocarbamate, diethyldithiocarbamate-s-glucuronide, inorganic sulfate and carbon disulfide were identified; these are also metabolites of disulfiram.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V12 221 (1976)
Although the ability of disulfiram to inactivate CYP2E1 has been known for more than 20 years, the mechanism has not yet been elucidated. A metabolite of disulfiram, diethyldithocarbamate (DDC), is converted by CYP2E1 to a reactive intermediate that subsequently inactivates the protein, leading to mechanism-based inactivation. Mass spectral analysis of the inactivated human 2E1 protein demonstrates that the inactivation is due to the formation of an adduct of the reactive metabolite of DDC with the apoprotein. These data, along with mass spectral analysis of a reactive intermediate trapped with GSH, indicate the involvement of a reactive intermediate with a molecular mass of 116 Da. Our results suggest that this binding involves formation of a disulfide bond with one of the eight cysteines in CYP2E1. The inactivation of wild-type CYP2E1 as well as two of its polymorphic mutants, CYP2E1*2 and CYP2E1*4, was also investigated. For wild-type CYP2E1, the K(I) was 12.2 uM and the k(inact) was 0.02 min(-1). The K(I) values for the two polymorphic mutants were 227.6 and 12.4 uM for CYP2E1.2 and CYP2E1.4, and the k(inact) values were 0.0061 and 0.0187 min(-1), respectively. These data indicate that DDC is a much less efficient inactivator of CYP2E1.2 than it is of either the wild-type or the CYP2E1.4 variant.
PMID:20826547 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2993453 Pratt-Hyatt M et al; Drug Metab Dispos 38 (12): 2286-92 (2010)
... DDTC significantly inhibited the activity of superoxide dismutase and the activity of gamma-glutamyl transpeptidase, glutathione reductase, and alkaline phosphatase, whereas an increase in the activity of glutathione peroxidase was found. The membranes of pneumocytes type II were injured.
PMID:11212946 Tatrai E et al; J Toxicol Environ Health A 62 (3): 207-16 (2001)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
ABOUT THIS PAGE
20
PharmaCompass offers a list of Ditiocarb API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ditiocarb manufacturer or Ditiocarb supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ditiocarb manufacturer or Ditiocarb supplier.
PharmaCompass also assists you with knowing the Ditiocarb API Price utilized in the formulation of products. Ditiocarb API Price is not always fixed or binding as the Ditiocarb Price is obtained through a variety of data sources. The Ditiocarb Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ditiocarb manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ditiocarb, including repackagers and relabelers. The FDA regulates Ditiocarb manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ditiocarb API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Ditiocarb supplier is an individual or a company that provides Ditiocarb active pharmaceutical ingredient (API) or Ditiocarb finished formulations upon request. The Ditiocarb suppliers may include Ditiocarb API manufacturers, exporters, distributors and traders.
click here to find a list of Ditiocarb suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Ditiocarb DMF (Drug Master File) is a document detailing the whole manufacturing process of Ditiocarb active pharmaceutical ingredient (API) in detail. Different forms of Ditiocarb DMFs exist exist since differing nations have different regulations, such as Ditiocarb USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Ditiocarb DMF submitted to regulatory agencies in the US is known as a USDMF. Ditiocarb USDMF includes data on Ditiocarb's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Ditiocarb USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Ditiocarb suppliers with USDMF on PharmaCompass.
Ditiocarb Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ditiocarb GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ditiocarb GMP manufacturer or Ditiocarb GMP API supplier for your needs.
A Ditiocarb CoA (Certificate of Analysis) is a formal document that attests to Ditiocarb's compliance with Ditiocarb specifications and serves as a tool for batch-level quality control.
Ditiocarb CoA mostly includes findings from lab analyses of a specific batch. For each Ditiocarb CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ditiocarb may be tested according to a variety of international standards, such as European Pharmacopoeia (Ditiocarb EP), Ditiocarb JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ditiocarb USP).

