



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
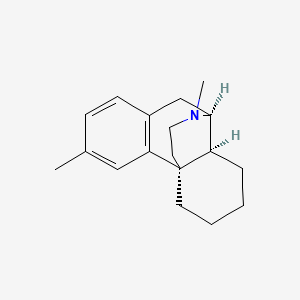

1. Astomin
2. At 17
3. D-3-methyl-n-methylmorphinan
4. Dastosin
5. Dimemorfan Phosphate
6. Dimemorfan Phosphate (1:1) Salt
7. Dimemorfan, (9alpha,13alpha,14alpha)-isomer
8. Dinemorphan
1. Dimemorfan [inn]
2. 3,17-dimethylmorphinan
3. 36309-01-0
4. 36304-82-2
5. (+)-3,17-dimethylmorphinan
6. 623oac38yu
7. Dimemorfane
8. Dimemorphan
9. Dimemorfano
10. Dimemorfanum
11. (1s,9s,10s)-4,17-dimethyl-17-azatetracyclo[7.5.3.01,10.02,7]heptadeca-2(7),3,5-triene
12. D-3-methyl-n-methylmorphinan
13. 3,n-dimethylmorphinan
14. N,3-dimethylmorphinan
15. At 17
16. Dimemorfane [inn-french]
17. Dimemorfanum [inn-latin]
18. Dimemorfano [inn-spanish]
19. Unii-623oac38yu
20. 3,n-dimethylmorphinan [iupac]
21. Einecs 252-963-3
22. Dimemorfan [mi]
23. Dimemorfan [who-dd]
24. Schembl499349
25. Chembl2106325
26. Chebi:135048
27. Dtxsid301043335
28. Zinc4215661
29. Db13810
30. Q5277240
31. Morphinan, 3,17-dimethyl-, (9alpha,13alpha,14alpha)-
| Molecular Weight | 255.4 g/mol |
|---|---|
| Molecular Formula | C18H25N |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 255.198699802 g/mol |
| Monoisotopic Mass | 255.198699802 g/mol |
| Topological Polar Surface Area | 3.2 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 352 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antitussive Agents
Agents that suppress cough. They act centrally on the medullary cough center. EXPECTORANTS, also used in the treatment of cough, act locally. (See all compounds classified as Antitussive Agents.)
R - Respiratory system
R05 - Cough and cold preparations
R05D - Cough suppressants, excl. combinations with expectorants
R05DA - Opium alkaloids and derivatives
R05DA11 - Dimemorfan


