
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
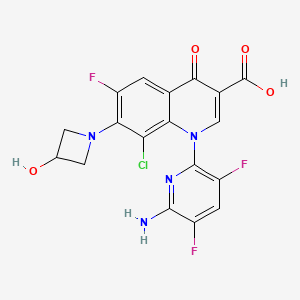

1. 1-(6-amino-3,5-difluoropyridin-2-yl)-8-chloro-6-fluoro-7-(3-hydroxyazetidin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid
2. 3-quinolinecarboxylic Acid, 1-(6-amino-3,5-difluoro-2-pyridinyl)-8-chloro-6-fluoro-1,4-dihydro-7-(3-hydroxy-1-azetidinyl)-4-oxo-
3. Abt 492
4. Abt-492
5. Abt492
6. Baxdela
7. Rx-3341
1. 189279-58-1
2. Abt-492
3. Baxdela
4. Wq-3034
5. Abt492
6. Abt 492
7. 1-(6-amino-3,5-difluoropyridin-2-yl)-8-chloro-6-fluoro-7-(3-hydroxyazetidin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid
8. Rx-3341
9. 1-(6-amino-3,5-difluoropyridin-2-yl)-8-chloro-6-fluoro-7-(3-hydroxyazetidin-1-yl)-4-oxoquinoline-3-carboxylic Acid
10. Delafloxacin [usan]
11. 3-quinolinecarboxylic Acid, 1-(6-amino-3,5-difluoro-2-pyridinyl)-8-chloro-6-fluoro-1,4-dihydro-7-(3-hydroxy-1-azetidinyl)-4-oxo-
12. 6315412yvf
13. Delafloxacin (usan)
14. 1-(6-amino-3,5-difluoro-2-pyridyl)-8-chloro-6-fluoro-7-(3-hydroxyazetidin-1-yl)-4-oxo-quinoline-3-carboxylic Acid
15. Delafloxacin [usan:inn]
16. Delafloxacinum
17. Quofenix
18. Unii-6315412yvf
19. Abbott 492
20. Delafloxacinabt-492
21. Delafloxacin [mi]
22. Delafloxacin [inn]
23. Delafloxacin [who-dd]
24. Schembl294694
25. Chembl2105637
26. Gtpl10799
27. Amy4217
28. Delafloxacin, >=98% (hplc)
29. Dtxsid40172331
30. Ex-a2331
31. Zinc3827556
32. Bdbm50560872
33. S1553
34. Cs-1478
35. Db11943
36. Sb16746
37. Wq 3034
38. Ncgc00390187-01
39. Ncgc00390187-02
40. Ac-30739
41. As-75147
42. Hy-14814
43. Delafloxacin 100 Microg/ml In Acetonitrile
44. Ft-0697895
45. C73623
46. D09330
47. Rx-3341; Wq-3034; Abt492; Delafloxacin
48. Abt-492(delafloxacin, Rx-3341, Wq-3034)
49. A-319492
50. Q5253067
51. 189279-58-1, 339591-82-1 (sodium Salt)
52. 1-(6-amino-3,5-difluoropyridine-2-yl)-8-chloro-6-fluoro-7-(3-hydroxyazetidine-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid
| Molecular Weight | 440.8 g/mol |
|---|---|
| Molecular Formula | C18H12ClF3N4O4 |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 3 |
| Exact Mass | 440.0499171 g/mol |
| Monoisotopic Mass | 440.0499171 g/mol |
| Topological Polar Surface Area | 120 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 755 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 1 | |
|---|---|
| Drug Name | BAXDELA |
| Active Ingredient | DELAFLOXACIN MEGLUMINE |
| Company | MELINTA THERAPS INC (Application Number: N208610); MELINTA THERAPS INC (Application Number: N208611) |
Delafloxacin is indicated for the treatment of acute bacterial skin and skin structure infections caused by the Gram-positive organisms Staphylococcus aureus (including methicillin-resistant and methicillin-susceptible isolates), Staphylococcus haemolyticus, Staphylococcus lugdunensis, Streptococcus agalactiae, Streptococcus anginosus Group (including Streptococcus anginosus, Streptococcus intermedius, and Streptococcus constellatus), Streptococcus pyogenes, and Enterococcus faecalis as well as the Gram-negative organisms Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae, and Pseudomonas aeruginosa.
FDA Label
Quofenix is indicated for the treatment of the following infections in adults:
- acute bacterial skin and skin structure infections (ABSSSI),
- community-acquired pneumonia (CAP), when it is considered inappropriate to use other antibacterial agents that are commonly recommended for the initial treatment of these infections (see sections 4. 4 and 5. 1).
Consideration should be given to official guidance on the appropriate use of antibacterial agents.
Treatment of community-acquired pneumonia
Treatment of local infections of skin and subcutaneous tissues
Treatment of local infections of skin and subcutaneous tissues
Delafloxacin is a fluoroquinolone antibacterial drug which kills bacterial cells.
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
J01MA23
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01M - Quinolone antibacterials
J01MA - Fluoroquinolones
J01MA23 - Delafloxacin
Absorption
The median time to peak plasma concentration for orally administered Delafloxacin is 0.75 (0.5-4.0) hours after a single dose and 1.00 (0.5-6.0) hours for steady state dosing. The median time to peak plasma concentration for intravenously administered Delafloxacin is 1.00 (1.0-1.2) hours for a single dose and 1.0 (1.0-1.0) hour for steady state dosing. The absolute bioavailability for orally administed Delafloxacin is 58.8%.
Route of Elimination
After a single intravenous dose, 65% of Delafloxacin was excreted in the urine either unchanged or as glucuronide metabolites with 28% excreted unchanged in the feces. After a single oral dose, 50% of Delafloxacin was excreted in the urine either unchanged or as glucuronide metabolites with 48% excreted unchanged in the feces.
Volume of Distribution
The steady sate volume of distrubution of Delafloxacin is 30-48 liters.
Clearance
The mean total clearance of Delafloxacin is 16.3 liters per hour. Renal clearance accounts for 35-45% of total clearance.
Delafoxacin is primarily metabolized via glucuronidation mediated by UDP glucuronosyltransferase 1-1, UDP-glucuronosyltransferase 1-3, and UDP-glucuronosyltransferase 2B15. Less than 1% is metabolized via oxidation.
The mean half life of elimination of Delafloxacin is 3.7 hours after a single intravenous administration. The mean half life of elimination for multple oral administrations is 4.2-8.5 hours.
Delafloxacin inhibits the activity of bacterial DNA topoisomerase IV and DNA gyrase (topoisomerase II). This interferes with bacterial DNA replication by preventing the relaxation of positive supercoils introduced as part of the elongation process. The resultant strain inhibits further elongation. Delafloxacin exerts concentration-dependent bacteriocidal activity.
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
About the Company : Established in 2004, Metrochem API is one of the fastest-growing APIs, pellets & intermediates manufacturers. It has 6 dedicated manufacturing facilities for its 3 core product gro...
 Lewens Labs transforms healthcare with innovative, sustainable, and affordable pharmaceutical solutions worldwide.
Lewens Labs transforms healthcare with innovative, sustainable, and affordable pharmaceutical solutions worldwide.
About the Company : Lewens Labs was established with a vision to transform the pharmaceutical industry, inspired by an award-winning engineering project that reduced the cost of Aceclofenac and receiv...
About the Company : Established in 2011 and situated in Hangzhou, Zhejiang, China, Hangzhou Longshine Bio-Tech CO., Ltd is dedicated to providing services for pharmaceutical and chemical products, cat...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Delafloxacin is a Antibiotic drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of undefined medical condition.
Lead Product(s): Delafloxacin,Inapplicable
Therapeutic Area: Undisclosed Brand Name: Undisclosed
Study Phase: Phase IProduct Type: Antibiotic
Sponsor: Biomedical Advanced Research and Development Authority
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 25, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Delafloxacin,Inapplicable
Therapeutic Area : Undisclosed
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Biomedical Advanced Research and Development Authority
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Delafloxacin is a Antibiotic drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of undefined medical condition.
Product Name : Undisclosed
Product Type : Antibiotic
Upfront Cash : Inapplicable
September 25, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The partnership aims to advance two antibiotics currently FDA-approved for adults, Baxdela (delafloxacin) and Vabomere (meropenem and vaborbactam), for use in pediatrics and also for the development of Baxdela against biothreat pathogens for both adults and children.
Lead Product(s): Delafloxacin,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Baxdela
Study Phase: Approved FDFProduct Type: Antibiotic
Sponsor: BARDA
Deal Size: $141.9 million Upfront Cash: Undisclosed
Deal Type: Partnership July 10, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Delafloxacin,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : BARDA
Deal Size : $141.9 million
Deal Type : Partnership
Details : The partnership aims to advance two antibiotics currently FDA-approved for adults, Baxdela (delafloxacin) and Vabomere (meropenem and vaborbactam), for use in pediatrics and also for the development of Baxdela against biothreat pathogens for both adults ...
Product Name : Baxdela
Product Type : Antibiotic
Upfront Cash : Undisclosed
July 10, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Under the terms of the agreement, Xediton is responsible for the registration and commercialization of BAXDELA® (delafloxacin), KIMYRSA® (oritavancin), ORBACTIV® (oritavancin) and VABOMERE® (meropenem and vaborbactam), four novel anti-infective products in Canada.
Lead Product(s): Delafloxacin,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Baxdela
Study Phase: Approved FDFProduct Type: Antibiotic
Sponsor: Xediton Pharmaceuticals
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Licensing Agreement May 15, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Delafloxacin,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Xediton Pharmaceuticals
Deal Size : Undisclosed
Deal Type : Licensing Agreement
Details : Under the terms of the agreement, Xediton is responsible for the registration and commercialization of BAXDELA® (delafloxacin), KIMYRSA® (oritavancin), ORBACTIV® (oritavancin) and VABOMERE® (meropenem and vaborbactam), four novel anti-infective produ...
Product Name : Baxdela
Product Type : Antibiotic
Upfront Cash : Undisclosed
May 15, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Menarini Ricerche has published four abstracts presenting the latest findings related to its meropenem/vaborbactam (Vaborem™) and delafloxacin (Quofenix™) clinical studies, confirming Menarini’s commitment to fighting life-threatening bacterial infections.
Lead Product(s): Delafloxacin,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Quofenix
Study Phase: Phase IIIProduct Type: Antibiotic
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable July 09, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Delafloxacin,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Menarini Ricerche Announces the Latest Findings of Its Vaborem™ and Quofenix™ Clinical Studies
Details : Menarini Ricerche has published four abstracts presenting the latest findings related to its meropenem/vaborbactam (Vaborem™) and delafloxacin (Quofenix™) clinical studies, confirming Menarini’s commitment to fighting life-threatening bacterial inf...
Product Name : Quofenix
Product Type : Antibiotic
Upfront Cash : Inapplicable
July 09, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Delafloxacin is a Antibiotic drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Surgical Wound Infection.
Lead Product(s): Delafloxacin,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Antibiotic
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable August 01, 2019

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Delafloxacin,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Delafloxacin is a Antibiotic drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Surgical Wound Infection.
Product Name : Undisclosed
Product Type : Antibiotic
Upfront Cash : Inapplicable
August 01, 2019

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Delafloxacin is a Antibiotic drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Pneumonia.
Lead Product(s): Delafloxacin,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Undisclosed
Study Phase: Phase IProduct Type: Antibiotic
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 23, 2018

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Delafloxacin,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Delafloxacin is a Antibiotic drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Pneumonia.
Product Name : Undisclosed
Product Type : Antibiotic
Upfront Cash : Inapplicable
May 23, 2018

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Delafloxacin is a Antibiotic drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Community-acquired Pneumonia, Bacterial.
Lead Product(s): Delafloxacin,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Antibiotic
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable February 10, 2016

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Delafloxacin,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Delafloxacin is a Antibiotic drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Community-acquired Pneumonia, Bacterial.
Product Name : Undisclosed
Product Type : Antibiotic
Upfront Cash : Inapplicable
February 10, 2016

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Midazolam is a Controlled Substance drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of undefined medical condition.
Lead Product(s): Midazolam,Delafloxacin
Therapeutic Area: Undisclosed Brand Name: Undisclosed
Study Phase: Phase IProduct Type: Controlled Substance
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable July 22, 2015

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Midazolam,Delafloxacin
Therapeutic Area : Undisclosed
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Midazolam is a Controlled Substance drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of undefined medical condition.
Product Name : Undisclosed
Product Type : Controlled Substance
Upfront Cash : Inapplicable
July 22, 2015

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Delafloxacin is a Antibiotic drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Liver Failure.
Lead Product(s): Delafloxacin,Inapplicable
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: Undisclosed
Study Phase: Phase IProduct Type: Antibiotic
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 19, 2014

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Delafloxacin,Inapplicable
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Pharmacokinetics of Delafloxacin in Subjects With and Without Hepatic Impairment
Details : Delafloxacin is a Antibiotic drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Liver Failure.
Product Name : Undisclosed
Product Type : Antibiotic
Upfront Cash : Inapplicable
September 19, 2014

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Delafloxacin is a Antibiotic drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Gonorrhea.
Lead Product(s): Delafloxacin,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Antibiotic
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 19, 2013

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Delafloxacin,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Comparison of Delafloxacin Versus Ceftriaxone for the Treatment of Uncomplicated Gonorrhea
Details : Delafloxacin is a Antibiotic drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Gonorrhea.
Product Name : Undisclosed
Product Type : Antibiotic
Upfront Cash : Inapplicable
December 19, 2013

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]3,5-Difluoro-2,6-pyridinediaminedihydroxybutanedio...
CAS Number : 247069-27-8
End Use API : Delafloxacin
About The Company : Established in 2011 and situated in Hangzhou, Zhejiang, China, Hangzhou Longshine Bio-Tech CO., Ltd is dedicated to providing services for pharmaceutical and ch...

3-Hydroxyazetidine hydrochloride
CAS Number : 18621-18-6
End Use API : Delafloxacin
About The Company : Established in 2011 and situated in Hangzhou, Zhejiang, China, Hangzhou Longshine Bio-Tech CO., Ltd is dedicated to providing services for pharmaceutical and ch...

Ethyl 3 -(3 -chloro-2,4,5-trifluorophenyl)-3 -oxop...
CAS Number : 101987-86-4
End Use API : Delafloxacin
About The Company : Established in 2011 and situated in Hangzhou, Zhejiang, China, Hangzhou Longshine Bio-Tech CO., Ltd is dedicated to providing services for pharmaceutical and ch...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

ABOUT THIS PAGE
28
PharmaCompass offers a list of Delafloxacin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Delafloxacin manufacturer or Delafloxacin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Delafloxacin manufacturer or Delafloxacin supplier.
PharmaCompass also assists you with knowing the Delafloxacin API Price utilized in the formulation of products. Delafloxacin API Price is not always fixed or binding as the Delafloxacin Price is obtained through a variety of data sources. The Delafloxacin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Delafloxacin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Delafloxacin, including repackagers and relabelers. The FDA regulates Delafloxacin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Delafloxacin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Delafloxacin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Delafloxacin supplier is an individual or a company that provides Delafloxacin active pharmaceutical ingredient (API) or Delafloxacin finished formulations upon request. The Delafloxacin suppliers may include Delafloxacin API manufacturers, exporters, distributors and traders.
click here to find a list of Delafloxacin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Delafloxacin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Delafloxacin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Delafloxacin GMP manufacturer or Delafloxacin GMP API supplier for your needs.
A Delafloxacin CoA (Certificate of Analysis) is a formal document that attests to Delafloxacin's compliance with Delafloxacin specifications and serves as a tool for batch-level quality control.
Delafloxacin CoA mostly includes findings from lab analyses of a specific batch. For each Delafloxacin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Delafloxacin may be tested according to a variety of international standards, such as European Pharmacopoeia (Delafloxacin EP), Delafloxacin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Delafloxacin USP).

