



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
0
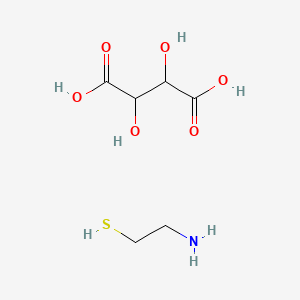

1. 2 Aminoethanethiol
2. 2-aminoethanethiol
3. 35s-labeled Cysteamine
4. Becaptan
5. Beta Mercaptoethylamine
6. Beta-mercaptoethylamine
7. Bitartrate, Cysteamine
8. Cystagon
9. Cysteamine
10. Cysteamine Dihydrochloride
11. Cysteamine Hydrobromide
12. Cysteamine Hydrochloride
13. Cysteamine Maleate (1:1)
14. Cysteamine Tartrate
15. Cysteamine Tartrate (1:1)
16. Cysteamine Tosylate
17. Cysteamine, 35s Labeled
18. Cysteamine, 35s-labeled
19. Cysteinamine
20. Dihydrochloride, Cysteamine
21. Hydrobromide, Cysteamine
22. Hydrochloride, Cysteamine
23. Mercamine
24. Mercaptamine
25. Mercaptoethylamine
26. Tartrate, Cysteamine
27. Tosylate, Cysteamine
1. 27761-19-9
2. Cysteamine (bitartrate)
3. Mercamine Bitartrate
4. Chebi:50386
5. Rp-103
6. Schembl49513
7. Chembl2062263
8. Bcp15048
9. Akos024332936
10. 2-aminoethanethiol 2,3-dihydroxysuccinate
11. Db-114321
12. Ft-0696891
13. 2-aminoethanethiol;2,3-dihydroxybutanedioic Acid
14. A900934
15. Q27122045
| Molecular Weight | 227.24 g/mol |
|---|---|
| Molecular Formula | C6H13NO6S |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 4 |
| Exact Mass | 227.04635831 g/mol |
| Monoisotopic Mass | 227.04635831 g/mol |
| Topological Polar Surface Area | 142 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 144 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Cystine Depleting Agents
Compounds and drugs that react with CYSTINE and convert it into a compound that can be more easily metabolized or intracellularly transported. Drugs in this class have been used to treat CYSTINOSIS. (See all compounds classified as Cystine Depleting Agents.)








