
Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Canada
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Aquacel
2. Aquaplast
3. Carboxymethyl Cellulose
4. Carboxymethylcellulose
5. Carboxymethylcellulose Sodium
6. Carboxymethylcellulose, Sodium
7. Carmellose Sodium
8. Cellolax
9. Cellulose, Carboxymethyl
10. Cethylose
11. Polycell
12. Ruspol
13. Sodium Carboxymethylcellulose
14. Sodium, Carboxymethylcellulose
15. Sodium, Carmellose
16. Sodium, Croscarmellose
1. Edifas B
2. Carboxymethylcellulose Sodium Salt
3. Cellulose Gum
4. 9004-32-4
5. Carboxymethyl Cellulose, Sodium Salt
6. 9085-26-1
7. Aquacel
8. Carmethose
9. Cellofas
10. Cellpro
11. Cellufresh
12. Cellugel
13. Celluvisc
14. Collowel
15. Ethoxose
16. Lovosa
17. Camellose Gum
18. Carmellose Gum
19. Sarcell Tel
20. Carboxymethylcellulose Sodium [usp]
21. Cellofas B
22. Cellofas C
23. Cellogel C
24. Carmellose Sodium
25. Cellogen Pr
26. Glikocel Ta
27. Cmc Sodium Salt
28. Nymcel S
29. Tylose C
30. Blanose Bwm
31. Nymcel Slc-t
32. Lovosa Tn
33. Tylose Cb Series
34. Tylose Cr
35. Unisol Rh
36. Cellofas B5
37. Cellofas B6
38. Cellogen 3h
39. Sodium Cmc
40. Tylose Dkl
41. Carbose 1m
42. Cellogen Ws-c
43. Majol Plx
44. Cellofas B50
45. Courlose F 4
46. Courlose F 8
47. Polyfibron 120
48. Tylose Cbr Series
49. Avicel Rc/cl
50. Fine Gum Hes
51. Nacm-cellulose Salt
52. Sodium Cm-cellulose
53. Courlose F 20
54. Sodium Carboxymethylcellulose
55. Copagel Pb 25
56. Sanlose Sn 20a
57. Cellufix Ff 100
58. Courlose A 590
59. Courlose A 610
60. Courlose A 650
61. Courlose F 370
62. Modocoll 1200
63. Nymcel Zsb 10
64. Nymcel Zsb 16
65. Tylose Cbs 30
66. Tylose Cbs 70
67. Tylose Cr 50
68. Blanose Bs 190
69. Tylose 666
70. Tylose C 30
71. Ac-di-sol. Nf
72. Lucel (polysaccharide)
73. Tylose Cbr 400
74. Courlose F 1000g
75. Tylose C 300
76. Tylose C 600
77. Tylose Cb 200
78. Daicel 1150
79. Daicel 1180
80. Tylose C 1000p
81. B 10 (polysaccharide)
82. Cm-cellulose Sodium Salt
83. Cellulose Sodium Glycolate
84. Sodium Cellulose Glycolate
85. Sodium Glycolate Cellulose
86. Cmc 7mt
87. Cmc 7h
88. Cmc 7h3sf
89. Cmc 7m
90. Sodium Carboxmethylcellulose
91. Cmc 3m5t
92. 7h3sf
93. Cmc 2
94. Aku-w 515
95. Kmts 212
96. Kmts 300
97. Kmts 500
98. Kmts 600
99. Cmc 41a
100. Cmc 4h1
101. Cmc 4m6
102. Cmc 7l1
103. Lovosa 20alk.
104. Ccris 3653
105. Cellulose Carboxymethyl Ether Sodium Salt
106. S 75m
107. Unii-e0dnv5jjhx
108. Cellulose Glycolic Acid, Sodium Salt
109. Sodium Salt Of Carboxymethylcellulose
110. Unii-6zq8v6yvnk
111. Unii-6yyv7vre59
112. Unii-72qqr5ryu4
113. Unii-d7sxm450nr
114. Unii-fc40a8xaj3
115. Unii-m8vp63k8fu
116. Unii-ryz9shl900
117. Unii-y3r0ra1q8s
118. Unii-ygx74dke74
119. Unii-0z2r7og99l
120. Unii-8ux21m67ij
121. Unii-8w25ji0g3v
122. Unii-m9j9397qws
123. Unii-r05y0b55jy
124. Unii-s5517jt8ys
125. Unii-v5u74hsl76
126. Unii-x075ft70ui
127. Unii-zy4732lp1o
128. B 10
129. Unii-0891bl4s3d
130. Unii-1rd48779fj
131. Unii-379m03vc9o
132. Unii-4j4p6l645m
133. Unii-75ku4500gf
134. Unii-97w605bik0
135. Unii-99h65d77xy
136. Unii-k679obs311
137. Unii-93o70285vh
138. Unii-kx442849t5
139. Carmellose Sodium, Low-substituted
140. Cellulose Carboxymethyl Ether, Sodium Salt
141. E0dnv5jjhx
142. 6zq8v6yvnk
143. Unii-0f4m8sis5k
144. 0f4m8sis5k
145. 6yyv7vre59
146. 72qqr5ryu4
147. D7sxm450nr
148. Fc40a8xaj3
149. M8vp63k8fu
150. Ryz9shl900
151. Y3r0ra1q8s
152. Ygx74dke74
153. Schembl454741
154. 0z2r7og99l
155. 8ux21m67ij
156. 8w25ji0g3v
157. M9j9397qws
158. R05y0b55jy
159. S5517jt8ys
160. V5u74hsl76
161. X075ft70ui
162. Zy4732lp1o
163. Refresh Plus, Cellufresh Formula
164. 0891bl4s3d
165. 1rd48779fj
166. 379m03vc9o
167. 4j4p6l645m
168. 75ku4500gf
169. 97w605bik0
170. 99h65d77xy
171. Cellulose, Carboxymethyl Ether, Sodium Salt, Low-substituted
172. K679obs311
173. Sodium Carboxymethylcellulose, Fcc
174. Akos015915206
175. 93o70285vh
176. Kx442849t5
177. Ft-0623482
178. A843419
179. Carboxymethylcellulose Sodium, Low-substituted
180. 117385-93-0
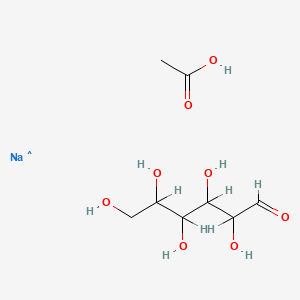
| Molecular Weight | 263.20 g/mol |
|---|---|
| Molecular Formula | C8H16NaO8 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 263.07428675 g/mol |
| Monoisotopic Mass | 263.07428675 g/mol |
| Topological Polar Surface Area | 156 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 169 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 4 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Laxatives
Agents that produce a soft formed stool, and relax and loosen the bowels, typically used over a protracted period, to relieve CONSTIPATION. (See all compounds classified as Laxatives.)
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 36002
Submission : 2021-06-08
Status : Active
Type : IV


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 34495
Submission : 2020-01-03
Status : Active
Type : IV


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 32790
Submission : 2018-04-27
Status : Active
Type : IV

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 11891
Submission : 1996-02-28
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 20279
Submission : 2007-02-05
Status : Inactive
Type : IV

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 2707
Submission : 1976-07-19
Status : Inactive
Type : II

USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 2876
Submission : 1977-02-24
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 9051
Submission : 1991-04-11
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

ABOUT THIS PAGE
60
PharmaCompass offers a list of Sodium Carboxymethylcellulose API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Sodium Carboxymethylcellulose manufacturer or Sodium Carboxymethylcellulose supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Sodium Carboxymethylcellulose manufacturer or Sodium Carboxymethylcellulose supplier.
PharmaCompass also assists you with knowing the Sodium Carboxymethylcellulose API Price utilized in the formulation of products. Sodium Carboxymethylcellulose API Price is not always fixed or binding as the Sodium Carboxymethylcellulose Price is obtained through a variety of data sources. The Sodium Carboxymethylcellulose Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A CMC 7M manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of CMC 7M, including repackagers and relabelers. The FDA regulates CMC 7M manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. CMC 7M API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of CMC 7M manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A CMC 7M supplier is an individual or a company that provides CMC 7M active pharmaceutical ingredient (API) or CMC 7M finished formulations upon request. The CMC 7M suppliers may include CMC 7M API manufacturers, exporters, distributors and traders.
click here to find a list of CMC 7M suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A CMC 7M DMF (Drug Master File) is a document detailing the whole manufacturing process of CMC 7M active pharmaceutical ingredient (API) in detail. Different forms of CMC 7M DMFs exist exist since differing nations have different regulations, such as CMC 7M USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A CMC 7M DMF submitted to regulatory agencies in the US is known as a USDMF. CMC 7M USDMF includes data on CMC 7M's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The CMC 7M USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of CMC 7M suppliers with USDMF on PharmaCompass.
A CMC 7M CEP of the European Pharmacopoeia monograph is often referred to as a CMC 7M Certificate of Suitability (COS). The purpose of a CMC 7M CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of CMC 7M EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of CMC 7M to their clients by showing that a CMC 7M CEP has been issued for it. The manufacturer submits a CMC 7M CEP (COS) as part of the market authorization procedure, and it takes on the role of a CMC 7M CEP holder for the record. Additionally, the data presented in the CMC 7M CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the CMC 7M DMF.
A CMC 7M CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. CMC 7M CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of CMC 7M suppliers with CEP (COS) on PharmaCompass.
CMC 7M Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of CMC 7M GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right CMC 7M GMP manufacturer or CMC 7M GMP API supplier for your needs.
A CMC 7M CoA (Certificate of Analysis) is a formal document that attests to CMC 7M's compliance with CMC 7M specifications and serves as a tool for batch-level quality control.
CMC 7M CoA mostly includes findings from lab analyses of a specific batch. For each CMC 7M CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
CMC 7M may be tested according to a variety of international standards, such as European Pharmacopoeia (CMC 7M EP), CMC 7M JP (Japanese Pharmacopeia) and the US Pharmacopoeia (CMC 7M USP).

