
Synopsis
Synopsis
0
JDMF
0
EU WC
0
VMF
0
Australia
0
South Africa
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
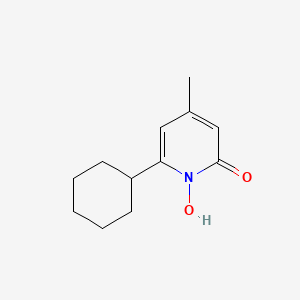

1. 6-cyclohexyl-1-hydroxy-4-methyl-2(1h)-pyridone Ethanolamine Salt
2. Batrafen
3. Ciclopirox Olamine
4. Ciclopiroxolamine
5. Cyclopirox
6. Cyclopyroxolamine
7. Dafnegin Csc
8. Dafnegin-csc
9. Hoe 296
10. Hoe-296
11. Hoe296
12. Loprox
13. Penlac
1. 29342-05-0
2. Loprox
3. Penlac
4. Hoe 296b
5. Cyclopirox
6. Ciclopiroxum
7. Hoe-296b
8. Ciclopiroxum [inn-latin]
9. 2(1h)-pyridinone, 6-cyclohexyl-1-hydroxy-4-methyl-
10. 6-cyclohexyl-1-hydroxy-4-methylpyridin-2(1h)-one
11. 6-cyclohexyl-1-hydroxy-4-methylpyridin-2-one
12. 6-cyclohexyl-1-hydroxy-4-methyl-2(1h)-pyridinone
13. Hoe296b
14. Ciclopirox (penlac)
15. Loprox (tn)
16. 19w019zdrj
17. Terit
18. Chebi:453011
19. 29342-05-0 (free)
20. Stieprox
21. Ciclopirox Gel
22. Loprox Cream
23. Loprox Gel
24. Ciclopirox Olamin
25. Ciclopirox-olamin
26. 6-cyclohexyl-4-methyl-1-oxidanyl-pyridin-2-one
27. 6-cyclohexyl-1-hydroxy-4-methyl-2(1h)-pyridone
28. 6-cyclohexyl-1-hydroxy-4-methyl-1h-pyridin-2-one
29. Mls002153867
30. Penlac (tn)
31. Ciclopirox (usp/inn)
32. Cnl8
33. Smr001233223
34. Einecs 249-577-2
35. Unii-19w019zdrj
36. Ciclodan
37. Ciclopirox [usan:usp:inn:ban]
38. 2(1h)-pyridone, 6-cyclohexyl-1-hydroxy-4-methyl-
39. Ciclopirox [mi]
40. Ciclopirox [inn]
41. Prestwick0_000541
42. Prestwick1_000541
43. Prestwick2_000541
44. Prestwick3_000541
45. Spectrum2_000146
46. Spectrum3_000351
47. Spectrum4_000288
48. Spectrum5_000747
49. Ciclopirox [usan]
50. Ciclopirox [vandf]
51. 6-cyclohexyl-1-hydroxy-4-methyl-pyridin-2-one
52. Ciclopirox [mart.]
53. Chembl1413
54. Ciclopirox [usp-rs]
55. Ciclopirox [who-dd]
56. Schembl34424
57. Bspbio_000581
58. Bspbio_002041
59. Kbiogr_000816
60. 6-cyclohexyl-1-hydroxy-4-methyl-2-(1h)-pyridone
61. Cid_38911
62. Bidd:gt0080
63. (6-cyclohexyl-1-hydroxy-4-methyl-2(1h)-pyridone)
64. Spbio_000252
65. Spbio_002502
66. Bpbio1_000641
67. Zinc1145
68. Ciclopirox [orange Book]
69. Ciclopirox, >=98% (hplc)
70. Dtxsid9048564
71. Bdbm66087
72. Gtpl11349
73. Kbio3_001261
74. Ciclopirox [ep Monograph]
75. Ciclopirox [usp Monograph]
76. Hms3656i12
77. Bcp28530
78. Hy-b0450
79. Mfcd00599441
80. S2528
81. Akos015895717
82. Ab06517
83. Ccg-266632
84. Db01188
85. Ks-5085
86. Ncgc00017112-04
87. Ncgc00017112-05
88. Ncgc00017112-06
89. Ncgc00017112-08
90. Ncgc00017112-11
91. Ncgc00178850-01
92. Ncgc00178850-02
93. Ac-24195
94. Ciclopirox 100 Microg/ml In Acetonitrile
95. Sbi-0206690.p002
96. Db-047566
97. Hoe 296; Hoe-296;ciclopiroxum; Penlac
98. 1-hydroxy-4-methyl-6-cyclohexyl-2-pyridone
99. Ft-0602961
100. Sw196923-5
101. 6-cyclohexyl-1-hydroxy-4-methyl-2-pyridinone
102. D03488
103. Ab00053438_09
104. Ab00053438_10
105. Ab00053438_11
106. 342c050
107. A819878
108. Q419468
109. Sr-05000001589-5
110. W-106995
111. 6-cyclohexyl-1-hydroxy-4-methyl-2(1h)-pyridinone #
112. Brd-k13044802-213-04-1
113. Brd-k13044802-213-09-0
114. 2-aminoethanol;6-cyclohexyl-1-hydroxy-4-methyl-2-pyridone
115. 6-cyclohexyl-1-hydroxy-4-methyl-1,2-dihydropyridin-2-one
116. Ciclopirox, European Pharmacopoeia (ep) Reference Standard
117. 2-aminoethanol;6-cyclohexyl-1-hydroxy-4-methyl-2-pyridinone
118. 2-azanylethanol;6-cyclohexyl-4-methyl-1-oxidanyl-pyridin-2-one
119. Ciclopirox, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 207.27 g/mol |
|---|---|
| Molecular Formula | C12H17NO2 |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 207.125928785 g/mol |
| Monoisotopic Mass | 207.125928785 g/mol |
| Topological Polar Surface Area | 40.5 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 325 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Ciclopirox |
| PubMed Health | Ciclopirox (On the skin) |
| Drug Classes | Antifungal |
| Drug Label | Ciclopirox Topical Solution, 8% (Nail Lacquer) contains a synthetic antifungal agent, ciclopirox. It is intended for topical use on fingernails and toenails and immediately adjacent skin.Each gram of Ciclopirox Topical Solution, 8% (Nail Lacquer) con... |
| Active Ingredient | Ciclopirox |
| Dosage Form | Gel; Shampoo; Cream; Solution; Suspension |
| Route | Topical |
| Strength | 8%; 1%; 0.77% |
| Market Status | Prescription |
| Company | Glenmark Generics; Fougera Pharms; Glenmark Pharms; Hi Tech Pharma; Paddock; Versapharm; Actavis Mid Atlantic; Perrigo; Perrigo New York; Taro Pharm Inds; Taro; Tolmar; G And W Labs; Cipla |
| 2 of 6 | |
|---|---|
| Drug Name | Loprox |
| PubMed Health | Ciclopirox (On the skin) |
| Drug Classes | Antifungal |
| Drug Label | LOPROX (ciclopirox) Shampoo 1% contains the synthetic antifungal agent, ciclopirox for topical use.Each gram (equivalent to 0.96 mL) of LOPROX Shampoo contains 10 mg ciclopirox in a shampoo base consisting of disodium laureth sulfosuccinate, laureth-... |
| Active Ingredient | Ciclopirox |
| Dosage Form | Shampoo; Cream; Suspension; Gel |
| Route | Topical |
| Strength | 1%; 0.77% |
| Market Status | Prescription |
| Company | Cnty Line Pharms; Medicis; Medimetriks Pharms |
| 3 of 6 | |
|---|---|
| Drug Name | Penlac |
| Drug Label | PENLAC NAIL LACQUER (ciclopirox) Topical Solution, 8%, contains a synthetic antifungal agent, ciclopirox. It is intended for topical use on fingernails and toenails and immediately adjacent skin. Each gram of PENLAC NAIL LACQUER (ciclopirox) Topi... |
| Active Ingredient | Ciclopirox |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 8% |
| Market Status | Prescription |
| Company | Valeant Bermuda |
| 4 of 6 | |
|---|---|
| Drug Name | Ciclopirox |
| PubMed Health | Ciclopirox (On the skin) |
| Drug Classes | Antifungal |
| Drug Label | Ciclopirox Topical Solution, 8% (Nail Lacquer) contains a synthetic antifungal agent, ciclopirox. It is intended for topical use on fingernails and toenails and immediately adjacent skin.Each gram of Ciclopirox Topical Solution, 8% (Nail Lacquer) con... |
| Active Ingredient | Ciclopirox |
| Dosage Form | Gel; Shampoo; Cream; Solution; Suspension |
| Route | Topical |
| Strength | 8%; 1%; 0.77% |
| Market Status | Prescription |
| Company | Glenmark Generics; Fougera Pharms; Glenmark Pharms; Hi Tech Pharma; Paddock; Versapharm; Actavis Mid Atlantic; Perrigo; Perrigo New York; Taro Pharm Inds; Taro; Tolmar; G And W Labs; Cipla |
| 5 of 6 | |
|---|---|
| Drug Name | Loprox |
| PubMed Health | Ciclopirox (On the skin) |
| Drug Classes | Antifungal |
| Drug Label | LOPROX (ciclopirox) Shampoo 1% contains the synthetic antifungal agent, ciclopirox for topical use.Each gram (equivalent to 0.96 mL) of LOPROX Shampoo contains 10 mg ciclopirox in a shampoo base consisting of disodium laureth sulfosuccinate, laureth-... |
| Active Ingredient | Ciclopirox |
| Dosage Form | Shampoo; Cream; Suspension; Gel |
| Route | Topical |
| Strength | 1%; 0.77% |
| Market Status | Prescription |
| Company | Cnty Line Pharms; Medicis; Medimetriks Pharms |
| 6 of 6 | |
|---|---|
| Drug Name | Penlac |
| Drug Label | PENLAC NAIL LACQUER (ciclopirox) Topical Solution, 8%, contains a synthetic antifungal agent, ciclopirox. It is intended for topical use on fingernails and toenails and immediately adjacent skin. Each gram of PENLAC NAIL LACQUER (ciclopirox) Topi... |
| Active Ingredient | Ciclopirox |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 8% |
| Market Status | Prescription |
| Company | Valeant Bermuda |
Used as a topical treatment in immunocompetent patients with mild to moderate onychomycosis of fingernails and toenails without lunula involvement, due to Trichophyton rubrum.
FDA Label
Ciclopirox is a broad-spectrum antifungal medication that also has antibacterial and anti-inflammatory properties. Its main mode of action is thought to be its high affinity for trivalent cations, which inhibit essential co-factors in enzymes. Ciclopirox exhibits either fungistatic or fungicidal activity in vitro against a broad spectrum of fungal organisms, such as dermatophytes, yeasts, dimorphic fungi, eumycetes, and actinomycetes. In addition to its broad spectrum of action, ciclopirox also exerts antibacterial activity against many Gram-positive and Gram-negative bacteria. Furthermore, the anti-inflammatory effects of ciclopirox have been demonstrated in human polymorphonuclear cells, where ciclopirox has inhibited the synthesis of prostaglandin and leukotriene. Ciclopirox can also exhibit its anti-inflammatory effects by inhibiting the formation of 5-lipoxygenase and cyclooxygenase.
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
D01AE14
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
D - Dermatologicals
D01 - Antifungals for dermatological use
D01A - Antifungals for topical use
D01AE - Other antifungals for topical use
D01AE14 - Ciclopirox
G - Genito urinary system and sex hormones
G01 - Gynecological antiinfectives and antiseptics
G01A - Antiinfectives and antiseptics, excl. combinations with corticosteroids
G01AX - Other antiinfectives and antiseptics
G01AX12 - Ciclopirox
Absorption
Rapidly absorbed after oral administration. Mean absorption of ciclopirox after application to nails of all twenty digits and adjacent 5 millimeters of skin once daily for 6 months in patients with dermatophytic onychomycoses was less than 5% of the applied dose. Ciclopirox olamine also penetrates into hair and through the epidermis and hair follicles into sebaceous glands and dermis.
Route of Elimination
Most of the compound is excreted either unchanged or as glucuronide. After oral administration of 10 mg of radiolabeled drug (14C-ciclopirox) to healthy volunteers, approximately 96% of the radioactivity was excreted renally within 12 hours of administration. Ninety-four percent of the renally excreted radioactivity was in the form of glucuronides.
Glucuronidation is the main metabolic pathway of ciclopirox.
1.7 hours for 1% topical solution.
Unlike antifungals such as itraconazole and terbinafine, which affect sterol synthesis, ciclopirox is thought to act through the chelation of polyvalent metal cations, such as Fe3+ and Al3+. These cations inhibit many enzymes, including cytochromes, thus disrupting cellular activities such as mitochondrial electron transport processes and energy production. Ciclopirox also appears to modify the plasma membrane of fungi, resulting in the disorganization of internal structures. The anti-inflammatory action of ciclopirox is most likely due to inhibition of 5-lipoxygenase and cyclooxygenase. ciclopirox may exert its effect by disrupting DNA repair, cell division signals and structures (mitotic spindles) as well as some elements of intracellular transport.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
27
PharmaCompass offers a list of Ciclopirox API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ciclopirox manufacturer or Ciclopirox supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ciclopirox manufacturer or Ciclopirox supplier.
PharmaCompass also assists you with knowing the Ciclopirox API Price utilized in the formulation of products. Ciclopirox API Price is not always fixed or binding as the Ciclopirox Price is obtained through a variety of data sources. The Ciclopirox Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ciclopirox manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ciclopirox, including repackagers and relabelers. The FDA regulates Ciclopirox manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ciclopirox API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ciclopirox manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ciclopirox supplier is an individual or a company that provides Ciclopirox active pharmaceutical ingredient (API) or Ciclopirox finished formulations upon request. The Ciclopirox suppliers may include Ciclopirox API manufacturers, exporters, distributors and traders.
click here to find a list of Ciclopirox suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Ciclopirox DMF (Drug Master File) is a document detailing the whole manufacturing process of Ciclopirox active pharmaceutical ingredient (API) in detail. Different forms of Ciclopirox DMFs exist exist since differing nations have different regulations, such as Ciclopirox USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Ciclopirox DMF submitted to regulatory agencies in the US is known as a USDMF. Ciclopirox USDMF includes data on Ciclopirox's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Ciclopirox USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Ciclopirox suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Ciclopirox Drug Master File in Korea (Ciclopirox KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Ciclopirox. The MFDS reviews the Ciclopirox KDMF as part of the drug registration process and uses the information provided in the Ciclopirox KDMF to evaluate the safety and efficacy of the drug.
After submitting a Ciclopirox KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Ciclopirox API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Ciclopirox suppliers with KDMF on PharmaCompass.
A Ciclopirox CEP of the European Pharmacopoeia monograph is often referred to as a Ciclopirox Certificate of Suitability (COS). The purpose of a Ciclopirox CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Ciclopirox EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Ciclopirox to their clients by showing that a Ciclopirox CEP has been issued for it. The manufacturer submits a Ciclopirox CEP (COS) as part of the market authorization procedure, and it takes on the role of a Ciclopirox CEP holder for the record. Additionally, the data presented in the Ciclopirox CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Ciclopirox DMF.
A Ciclopirox CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Ciclopirox CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Ciclopirox suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Ciclopirox as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Ciclopirox API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Ciclopirox as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Ciclopirox and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Ciclopirox NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Ciclopirox suppliers with NDC on PharmaCompass.
Ciclopirox Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ciclopirox GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ciclopirox GMP manufacturer or Ciclopirox GMP API supplier for your needs.
A Ciclopirox CoA (Certificate of Analysis) is a formal document that attests to Ciclopirox's compliance with Ciclopirox specifications and serves as a tool for batch-level quality control.
Ciclopirox CoA mostly includes findings from lab analyses of a specific batch. For each Ciclopirox CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ciclopirox may be tested according to a variety of international standards, such as European Pharmacopoeia (Ciclopirox EP), Ciclopirox JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ciclopirox USP).

