
Synopsis
0
KDMF
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
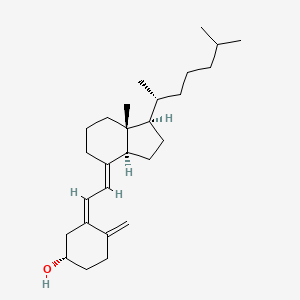

1. (3 Beta,5z,7e)-9,10-secocholesta-5,7,10(19)-trien-3-ol
2. Calciol
3. Cholecalciferols
4. Vitamin D 3
5. Vitamin D3
1. Vitamin D3
2. 67-97-0
3. Colecalciferol
4. Calciol
5. Oleovitamin D3
6. Ricketon
7. Arachitol
8. Trivitan
9. Deparal
10. Activated 7-dehydrocholesterol
11. Delsterol
12. Vigorsan
13. Ebivit
14. Vitamin D-3
15. Colecalcipherol
16. Quintox
17. Colecalciferolum
18. Cholecalciferolum
19. (+)-vitamin D3
20. D3-vicotrat
21. D3-vigantol
22. 1406-16-2
23. Vi-de-3-hydrosol
24. Neo Dohyfral D3
25. Vitinc Dan-dee-3
26. Cholecalciferol, D3
27. Vigantol
28. Vi-de3
29. Duphafral D3 1000
30. Feracol
31. Delta-d
32. Cc
33. Chebi:28940
34. (1s,3z)-3-[(2e)-2-[(1r,3as,7ar)-7a-methyl-1-[(2r)-6-methylheptan-2-yl]-2,3,3a,5,6,7-hexahydro-1h-inden-4-ylidene]ethylidene]-4-methylidenecyclohexan-1-ol
35. Nsc 375571
36. 9,10-secocholesta-5,7,10(19)-trien-3-beta-ol
37. Provitina
38. Micro-dee
39. 7-dehydrocholesterol, Activated
40. Vidde-3-hydrosol
41. Vitamin D3 Solution
42. Colecalciferol (inn)
43. Colecalciferol [inn]
44. Ncgc00159331-02
45. Rampage
46. Vitamin D3 10 Microg/ml In Acetonitrile
47. (3beta,5z,7e)-9,10-secocholesta-5,7,10(19)-trien-3-ol
48. (5z,7e)-(3s)-9,10-seco-5,7,10(19)-cholestatrien-3-ol
49. (5z,7e)-(3s)-9,10-secocholesta-5,7,10(19)-trien-3-ol
50. Dsstox_cid_6294
51. Dsstox_rid_78090
52. Dsstox_gsid_26294
53. Vitamin D3 (cholecalciferol)
54. 9,10-seco(5z,7e)-5,7,10(19)-cholestatrien-3beta-ol
55. Colecalciferolo
56. (3s,5z,7e)-9,10-secocholesta-5,7,10(19)-trien-3-ol
57. Colecalciferolo [dcit]
58. 9,10-secocholesta-5(z),7(e),10(19)-trien-3(.beta.)-ol
59. Vigantol Oil
60. (5e)-cholecalciferol
61. Nsc-375571
62. Colecalciferol D3
63. (1s,3z)-3-[(2e)-2-[(1r,3as,7ar)-1-[(1r)-1,5-dimethylhexyl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1h-inden-4-ylidene]ethylidene]-4-methylene-cyclohexanol
64. (s,z)-3-(2-((1r,3as,7ar,e)-7a-methyl-1-((r)-6-methylheptan-2-yl)octahydro-4h-inden-4-ylidene)ethylidene)-4-methylenecyclohexan-1-ol
65. Colecalciferolum [inn-latin]
66. Vitamin D 3
67. 7-dehydrocholestrol, Activated
68. Irradiated 7-dehydrocholesterol
69. Ccris 5813
70. Ccris 6286
71. Hsdb 820
72. Cholecalciferol Impurity A
73. 7-dehydrocholesterol, Irradiated
74. Vitamin D (cholecalciferol)
75. Vitamin D3 Emulsifiable
76. Einecs 200-673-2
77. Einecs 215-797-2
78. Mfcd00078131
79. Unii-9vu1ki44gp
80. Epa Pesticide Chemical Code 202901
81. Vitamin D3 (as Cholecalciferol)
82. Vitamin D3; Cholecalciferol
83. 1c6v77qf41
84. Devaron
85. Videkhol
86. Vitamin D Assay System Suitability
87. 7-dehydrocholesterol Activated
88. Unii-1c6v77qf41
89. Nsc375571
90. Granuvit D3
91. Dp-r206
92. Cas-67-97-0
93. Prestwick_63
94. Ak R215 Component Colecalciferol
95. Ak-r215 Component Colecalciferol
96. Cholecalciferol D3
97. Cyclohexanol, 3-((2e)-2-((1r,3as,7ar)-1-((1r)-1,5-dimethylhexyl)octahydro-7a-methyl-4h-inden-4-ylidene)ethylidene)-4-methylene-, (1s,3z)-
98. Cyclohexanol, 3-[(2e)-2-[(1r,3as,7ar)-1-[(1r)-1,5-dimethylhexyl]octahydro-7a-methyl-4h-inden-4-ylidene]ethylidene]-4-methylene-, (1s,3z)-
99. 9,10-secocholesta-5,7,10-trien-3-ol
100. Cholecalciferol [usp:ban:jan:iso]
101. ()-vitamin D3
102. 9,10-seco(5z,7e)-5,7,10(19)-cholestatrien-3-ol
103. Delta-d (tn)
104. 9,10-secocholesta-5,7,10(19)-trien-3-ol, (3beta,5z,7e)-
105. Prestwick3_000429
106. Bmse000507
107. Upcmld-dp152
108. Schembl3126
109. 9vu1ki44gp
110. Chembl1042
111. Bspbio_000418
112. Cholecalciferol; 67-97-0
113. Cholecalciferol (jp17/usp)
114. Bpbio1_000460
115. Megxm0_000458
116. Dtxsid6026294
117. Upcmld-dp152:001
118. Acon1_001997
119. Hms2096e20
120. Cholecalciferol, >=98% (hplc)
121. 9,10-secocholestra-5,7,10(19)-trien-3-ol, (3beta,5z,7e)-
122. Cholecalciferol, Analytical Standard
123. Zinc4474460
124. Tox21_111578
125. Tox21_202546
126. Bdbm50030475
127. Lmst03020001
128. S4063
129. Cholecalciferol For System Suitability
130. Akos015950641
131. Ac-8884
132. Ccg-268466
133. Cs-1179
134. Db00169
135. Smp1_000068
136. Vitamin D3 100 Microg/ml In Methanol
137. Ncgc00091072-01
138. Ncgc00159331-04
139. Ncgc00260095-01
140. Bs-42465
141. Hy-15398
142. Cholecalciferol (d3), Analytical Standard
143. C05443
144. D00188
145. 9,10-secocholesta-5,7,10(19)-trien-3-ol
146. Cholecalciferol, Meets Usp Testing Specifications
147. 078v131
148. 9,10-secocholesta-5,7,10(19)-trien-3?-ol
149. Q139347
150. (5e,7e)-9,10-secocholesta-5,7,10-trien-3-ol
151. Q-201931
152. 3-beta,z,7e-9,10-secocholestr-5,7,10(19)-trien-3-ol
153. Vitamin D3 Solution, 100 Mug/ml In Ethanol, 97% (cp)
154. (3beta,z,7e)-9,10-secocholesta-5,7,10(19)-trien-3-ol
155. 9,10-secocholesta-5,7,10(19)-trien-3-ol, (3b,5z,7e)-
156. Cholecalciferol, European Pharmacopoeia (ep) Reference Standard
157. Colecalciferol, British Pharmacopoeia (bp) Reference Standard
158. Cholecalciferol, United States Pharmacopeia (usp) Reference Standard
159. Cholecalciferol For System Suitability, European Pharmacopoeia (ep) Reference Standard
160. Vitamin D3 Solution, 1 Mg/ml In Ethanol, Ampule Of 1 Ml, Certified Reference Material
161. (1s,3z)-3-[(2e)-2-[7a-methyl-1-(6-methylheptan-2-yl)-2,3,3a,5,6,7-hexahydro-1h-inden-4-ylidene]ethylidene]-4-methylidenecyclohexan-1-ol
162. Cholecalciferol (vitamin D3), Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 384.6 g/mol |
|---|---|
| Molecular Formula | C27H44O |
| XLogP3 | 7.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 6 |
| Exact Mass | 384.339216023 g/mol |
| Monoisotopic Mass | 384.339216023 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 610 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Vitamin d |
| PubMed Health | Vitamin D |
| Drug Classes | Nutriceutical, Nutritive Agent, Vitamin Combination, Adult Formula |
| Active Ingredient | Ergocalciferol |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 50,000 iu |
| Market Status | Prescription |
| Company | Banner Pharmacaps |
| 2 of 2 | |
|---|---|
| Drug Name | Vitamin d |
| PubMed Health | Vitamin D |
| Drug Classes | Nutriceutical, Nutritive Agent, Vitamin Combination, Adult Formula |
| Active Ingredient | Ergocalciferol |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 50,000 iu |
| Market Status | Prescription |
| Company | Banner Pharmacaps |
Bone Density Conservation Agents; Vitamins
National Library of Medicine, SIS; ChemIDplus Record for Cholecalciferol (67-97-0), MESH Heading. Available from, as of March 15, 2006: https://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
MEDICATION (VET): Nutritional factor (Antirachitic)
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1787
Therapeutic doses of specific vitamin D analogs are used in the treatment of chronic hypocalcemia, hypophosphatemia, rickets, and osteodystrophy associated with various medical conditions including chronic renal failure, familial hypophosphatemia, and hypoparathyroidism (postsurgical or idiopathic, or pseudohypoparathyroidism). Some analogs have been found to reduct elevated parathyroid hormone concentrations in patients with renal osteodystrophy associated with hyperparathyroidism. Theoretically, any of the vitamin D analogs may be used for the above conditions, However, because of their pharmacologic properties, some may be more useful in certain situations than others. Alfacalcidol, calcitriol, and dihydrotachysterol are usually preferred in patients with renal failure since these patients have impaired ability to synthesize calcitriol from cholecalciferol and ergocalciferol; therefore, the response is more predictable. In addition, their shorter half-lives may make toxicity easier to manage (hypercalcemia reverses more quickly). Ergocalciferol may not be the preferred agent in the treatment of familial hypophosphatemia or hypoparathyroidism because the large doses needed are associated with a risk of overdose and hypercalcemia; dihydrotachysterol and calcitriol may be preferred. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2966
Studies have shown that the elderly may have an increased need for vitamin D due to a possible decrease in the capacity of the skin to produce previtamin D3 or a decrease in exposure to the sun or impaired renal function or impaired vitamin D absorption.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2967
Doses of vitamin D analogs that do not exceed the physiologic requirement are usually nontoxic. However, some infants and patients with sarcoidosis or hypoparathyroidism may have increased sensitivity to vitamin D analogs. /Vitamin D analogs/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3541
Acute or chronic administration of excessive doses of vitamin D analogs or enhanced responsiveness to physiologic amounts of ergocalciferol or cholecalciferol may lead to hypervitaminosis D manifested by hypercalcemia. /Vitamin D analogs/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3541
Decreased renal function without hypercalcemia has also been reported in patients with hypoparathyroidism after long-term vitamin D analog therapy. Before therapy with vitamin D analogs is initiated, serum phosphate concentrations must be controlled. To avoid ectopic calcification, the serum calcium (in mg/dL) times phosphorus (in mg/dL) should not be allowed to exceed 70. Because administration of vitamin D analogs may increase phosphate absorption, patients with renal failure may require adjustment in the dosage of aluminum-containing antacids used to decrease phosphate absorption. /Vitamin D analogs/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3541
For more Drug Warnings (Complete) data for CHOLECALCIFEROL (10 total), please visit the HSDB record page.
Cholecalciferol use is indicated for the treatment of specific medical conditions like refractory rickets (or vitamin D resistant rickets), hypoparathyroidism, and familial hypophosphatemia. Concurrently, as one of the most commonly utilized forms of vitamin D, cholecalciferol is also very frequently used as a supplement in individuals to maintain sufficient vitamin d levels in the body or to treat vitamin D deficiency, as well as various medical conditions that can be associated directly or indirectly with vitamin d insufficiency like osteoporosis and chronic kidney disease, among others.
Treatment of osteoporosis
The in vivo synthesis of the predominant two biologically active metabolites of vitamin D occurs in two steps. The first hydroxylation of vitamin D3 cholecalciferol (or D2) occurs in the liver to yield 25-hydroxyvitamin D while the second hydroxylation happens in the kidneys to give 1, 25-dihydroxyvitamin D. These vitamin D metabolites subsequently facilitate the active absorption of calcium and phosphorus in the small intestine, serving to increase serum calcium and phosphate levels sufficiently to allow bone mineralization. Conversely, these vitamin D metabolites also assist in mobilizing calcium and phosphate from bone and likely increase the reabsorption of calcium and perhaps also of phosphate via the renal tubules. There exists a period of 10 to 24 hours between the administration of cholecalciferol and the initiation of its action in the body due to the necessity of synthesis of the active vitamin D metabolites in the liver and kidneys. It is parathyroid hormone that is responsible for the regulation of such metabolism at the level of the kidneys.
Bone Density Conservation Agents
Agents that inhibit BONE RESORPTION and/or favor BONE MINERALIZATION and BONE REGENERATION. They are used to heal BONE FRACTURES and to treat METABOLIC BONE DISEASES such as OSTEOPOROSIS. (See all compounds classified as Bone Density Conservation Agents.)
Calcium-Regulating Hormones and Agents
Hormones and molecules with calcium-regulating hormone-like actions that modulate OSTEOLYSIS and other extra-skeletal activities to maintain calcium homeostasis. (See all compounds classified as Calcium-Regulating Hormones and Agents.)
Vitamins
Organic substances that are required in small amounts for maintenance and growth, but which cannot be manufactured by the human body. (See all compounds classified as Vitamins.)
A - Alimentary tract and metabolism
A11 - Vitamins
A11C - Vitamin a and d, incl. combinations of the two
A11CC - Vitamin d and analogues
A11CC05 - Colecalciferol
Absorption
Cholecalciferol is readily absorbed from the small intestine if fat absorption is normal. Moreover, bile is necessary for absorption as well. In particular, recent studies have determined aspects about the absorption of vitamin D, like the fact that a) the 25-hydroxyvitamin D metabolite of cholecalciferol is absorbed to a greater extent than the nonhydroxy form of cholecalciferol, b) the quantity of fat with which cholecalciferol is ingested does not appear to largely affect its bioavailability, and c) age does not apparently effect vitamin D cholecalciferol.
Route of Elimination
It has been observed that administered cholecalciferol and its metabolites are excreted primarily in the bile and feces.
Volume of Distribution
Studies have determined that the mean central volume of distribution of administered cholecalciferol supplementation in a group of 49 kidney transplant patients was approximately 237 L.
Clearance
Studies have determined that the mean clearance value of administered cholecalciferol supplementation in a group of 49 kidney transplant patients was approximately 2.5 L/day.
Readily absorbed from small intestine (proximal or distal); cholecalciferol may be absorbed more rapidly and completely than ergocalciferol.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2967
Elimination: Biliary/renal. /Vitamin D and analogs/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2967
Many vitamin D analogs are readily absorbed from the GI tract following oral administration if fat absorption is normal. The presence of bile is required for absorption of ergocalciferol and the extent of GI absorption may be decreased in patients with hepatic, biliary, or GI disease (e.g., Crohn's disease, Whipple's disease, sprue). Because vitamin D is fat soluble, it is incorporated into chylomicrons and absorbed via the lymphatic system; approximately 80% of ingested vitamin D appears to be absorbed systemically through this mechanism, principally in the small intestine. Although some evidence suggested that intestinal absorption of vitamin D may be decreased in geriatric adults, other evidence did not show clinically important age-related alterations in GI absorption of the vitamin in therapeutic doses. It currently is not known whether aging alters the GI absorption of physiologic amounts of vitamin D. /Vitamin D analogs/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3543
After absorption, ergocalciferol and cholecalciferol enter the blood via chylomicrons of lymph and then associate mainly with a specific alpha-globulin (vitamin D-binding protein). The hydroxylated metabolites of ergocalciferol and cholecalciferol also circulate associated with the same alpha-globulin. 25-Hydroxylated ergocalciferol and cholecalciferol are stored in fat and muscles for prolonged periods. Once vitamin D enters systemic circulation from lymph via the thoracic duct or from skin, it accumulates in the liver within a few hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3543
For more Absorption, Distribution and Excretion (Complete) data for CHOLECALCIFEROL (7 total), please visit the HSDB record page.
Within the liver, cholecalciferol is hydroxylated to calcifediol (25-hydroxycholecalciferol) by the enzyme vitamin D-25-hydroxylase. At the kidney, calcifediol subsequently serves as a substrate for 1-alpha-hydroxylase, yielding calcitriol (1,25-dihydroxycholecalciferol), the biologically active form of vitamin D3.
Metabolic activation of cholecalciferol and ergocalciferol occurs in 2 steps, the first in the liver and the second in the kidneys. Metabolic activation of calcifediol occurs in the kidneys; dihydrotachysterol, alfacalcidol and doxercalciferol are activated in the liver.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2967
Normal combined (ie, 25-hydroxyvitamin D) plasma concentrations of 25-hydroxycholecalciferol (calcifediol) and 25-hydroxyergocalciferol, which are the major circulating metabolites of cholecalciferol and ergocalciferol, have been reported to range from 8-80 ng/mL, depending on the assay used, and vary with exposure to UV light. A commonly reported range for the lower limit of normal is 8-15 ng/mL, depending on geographic location (eg, Southern California would be higher than Massachusetts).
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3542
In the liver, ergocalciferol and cholecalciferol are converted in the mitochondria to their 25-hydroxy derivatives by the enzyme vitamin D 25-hydroxylase. Vitamin D 25-hydroxylase activity is regulated in the liver by concentrations of vitamin D and its metabolites; therefore, increases in the systemic circulation of the 25-hydroxy metabolites following exposure to sunlight or ingestion of vitamin D are relatively modest compared with cumulative production or intake of the vitamin. Serum concentrations of nonhydroxylated vitamin D are short-lived as a result of storage in fat or metabolism in the liver. In the kidneys, these metabolites are further hydroxylated at the 1 position by the enzyme vitamin D 1-hydroxylase to their active forms, 1,25-dihydroxycholecalciferol (calcitriol) and 1,25-dihydroxyergocalciferol. ... Activity of the vitamin D 1-hydroxylase enzyme requires molecular oxygen, magnesium ion, and malate and is regulated principally by PTH in response to serum concentrations of calcium and phosphate, and perhaps by circulating concentrations of 1,25-dihydroxyergocalciferol and 1,25-dihydroxycholecalciferol. Other hormones (ie, cortisol, estrogens, prolactin, and growth hormone) also may influence the metabolism of cholecalciferol and ergocalciferol.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3543
The hepatic enzyme system responsible for 25-hydroxylation of vitamin D /(vitamin D-25 hydroxylase)/ is associated with the microsomal and mitochondrial fractions of homogenates and requires NADPH (nicotinamide adenine dinucleotide phosphate, reduced form) and molecular oxygen. ... The enzyme system /in kidney/ responsible for 1-hydroxylation of 25-OHD (25-hydroxycholecalciferol) /(25-OHD-1-alpha-hydroxylase)/ is associated with mitochondria in the proximal tubules. It is a mixed function oxidase and requires molecular oxygen and NADPH as cofactors. Cytochrome P450, a flavoprotein, and ferredoxin are components of the enzyme complex.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1727
At this time, there have been resources that document the half-life of cholecalciferol as being about 50 days while other sources have noted that the half-life of calcitriol (1,25-dihydroxyvitamin D3) is approximately 15 hours while that of calcidiol (25-hydroxyvitamin D3) is about 15 days. Moreover, it appears that the half-lives of any particular administration of vitamin d can vary due to variations in vitamin d binding protein concentrations and genotype in particular individuals.
The Vitamin /D/ disappears from plasma with a half-life of 19 to 25 hr but is stored in fat depots for prolonged periods. ... The 25-hydroxy derivative has a biological half-life of 19 days ... The plasma half-life of calcitriol /(1,25-dihydroxy-vitamin D)/ is estimated to be between 3 and 5 days in human beings ...
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1730
Most individuals naturally generate adequate amounts of vitamin D through ordinary dietary intake of vitamin D (in some foods like eggs, fish, and cheese) and natural photochemical conversion of the vitamin D3 precursor 7-dehydrocholesterol in the skin via exposure to sunlight. Conversely, vitamin D deficiency can often occur from a combination of insufficient exposure to sunlight, inadequate dietary intake of vitamin D, genetic defects with endogenous vitamin D receptor, or even severe liver or kidney disease. Such deficiency is known for resulting in conditions like rickets or osteomalacia, all of which reflect inadequate mineralization of bone, enhanced compensatory skeletal demineralization, resultant decreased calcium ion blood concentrations, and increases in the production and secretion of parathyroid hormone. Increases in parathyroid hormone stimulate the mobilization of skeletal calcium and the renal excretion of phosphorus. This enhanced mobilization of skeletal calcium leads towards porotic bone conditions. Ordinarily, while vitamin D3 is made naturally via photochemical processes in the skin, both itself and vitamin D2 can be found in various food and pharmaceutical sources as dietary supplements. The principal biological function of vitamin D is the maintenance of normal levels of serum calcium and phosphorus in the bloodstream by enhancing the efficacy of the small intestine to absorb these minerals from the diet. At the liver, vitamin D3 or D2 is hydroxylated to 25-hydroxyvitamin D and then finally to the primary active metabolite 1,25-dihydroxyvitamin D in the kidney via further hydroxylation. This final metabolite binds to endogenous vitamin d receptors, which results in a variety of regulatory roles - including maintaining calcium balance, the regulation of parathyroid hormone, the promotion of the renal reabsorption of calcium, increased intestinal absorption of calcium and phosphorus, and increased calcium and phosphorus mobilization of calcium and phosphorus from bone to plasma to maintain balanced levels of each in bone and the plasma. In particular, calcitriol interacts with vitamin D receptors in the small intestine to enhance the efficiency of intestinal calcium and phosphorous absorption from about 10-15% to 30-40% and 60% increased to 80%, respectively. Furthermore, calcitriol binds with vitamin D receptors in osteoblasts to stimulate a receptor activator of nuclear factor kB ligand (or RANKL) which subsequently interacts with receptor activator of nuclear factor kB (NFkB) on immature preosteoclasts, causing them to become mature bone-resorbing osteoclasts. Such mature osteoclasts ultimately function in removing calcium and phosphorus from bone to maintain blood calcium and phosphorus levels. Moreover, calcitriol also stimulates calcium reabsorption from the glomerular filtrate in the kidneys. Additionally, it is believed that when calcitriol binds with nuclear vitamin D receptors, that this bound complex itself binds to retinoic acid X receptor (RXR) to generate a heterodimeric complex that consequently binds to specific nucleotide sequences in the DNA called vitamin D response elements. When bound, various transcription factors attach to this complex, resulting in either up or down-regulation of the associated gene's activity. It is thought that there may be as much as 200 to 2000 genes that possess vitamin D response elements or that are influenced indirectly to control a multitude of genes across the genome. It is in this way that cholecalciferol is believed to function in regulating gene transcription associated with cancer risk, autoimmune disorders, and cardiovascular disease linked to vitamin D deficiency. In fact, there has been some research to suggest calcitriol may also be able to prevent malignancies by inducing cellular maturation and inducing apoptosis and inhibiting angiogenesis, exhibit anti-inflammatory effects by inhibiting foam cell formation and promoting angiogenesis in endothelial colony-forming cells in vitro, inhibit immune reactions by enhancing the transcription of endogenous antibiotics like cathelicidin and regulate the activity and differentiation of CD4+ T cells, amongst a variety of other proposed actions.
The principal biologic function of vitamin D is to maintain serum calcium and phosphorus concentrations within the normal range by enhancing the efficiency of the small intestine to absorb these minerals from the diet. Calcitriol (activated vitamin D) enhances the efficiency of intestinal calcium absorption along the entire small intestine, but principally in the duodenum and jejunum. Calcitriol also enhances phosphorus absorption along the entire small intestine, but principally in the jejunum and ileum. The activated forms of ergocalciferol, doxercalciferol, and cholecalciferol may have a negative feedback effect on parathyroid hormone (PTH) production.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3542
The most two important mechanisms by which vitamin D acts to maintain normal concentration of calcium and phosphate in plasma are to facilitate their absorption by the small intestine and to enhance their mobilization from bone. /Vitamin D/
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 1541

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
54
PharmaCompass offers a list of Vitamin D3 API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Vitamin D3 manufacturer or Vitamin D3 supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Vitamin D3 manufacturer or Vitamin D3 supplier.
PharmaCompass also assists you with knowing the Vitamin D3 API Price utilized in the formulation of products. Vitamin D3 API Price is not always fixed or binding as the Vitamin D3 Price is obtained through a variety of data sources. The Vitamin D3 Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Cholecalciferol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Cholecalciferol, including repackagers and relabelers. The FDA regulates Cholecalciferol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Cholecalciferol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Cholecalciferol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Cholecalciferol supplier is an individual or a company that provides Cholecalciferol active pharmaceutical ingredient (API) or Cholecalciferol finished formulations upon request. The Cholecalciferol suppliers may include Cholecalciferol API manufacturers, exporters, distributors and traders.
click here to find a list of Cholecalciferol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Cholecalciferol DMF (Drug Master File) is a document detailing the whole manufacturing process of Cholecalciferol active pharmaceutical ingredient (API) in detail. Different forms of Cholecalciferol DMFs exist exist since differing nations have different regulations, such as Cholecalciferol USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Cholecalciferol DMF submitted to regulatory agencies in the US is known as a USDMF. Cholecalciferol USDMF includes data on Cholecalciferol's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Cholecalciferol USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Cholecalciferol suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Cholecalciferol Drug Master File in Japan (Cholecalciferol JDMF) empowers Cholecalciferol API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Cholecalciferol JDMF during the approval evaluation for pharmaceutical products. At the time of Cholecalciferol JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Cholecalciferol suppliers with JDMF on PharmaCompass.
A Cholecalciferol CEP of the European Pharmacopoeia monograph is often referred to as a Cholecalciferol Certificate of Suitability (COS). The purpose of a Cholecalciferol CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Cholecalciferol EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Cholecalciferol to their clients by showing that a Cholecalciferol CEP has been issued for it. The manufacturer submits a Cholecalciferol CEP (COS) as part of the market authorization procedure, and it takes on the role of a Cholecalciferol CEP holder for the record. Additionally, the data presented in the Cholecalciferol CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Cholecalciferol DMF.
A Cholecalciferol CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Cholecalciferol CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Cholecalciferol suppliers with CEP (COS) on PharmaCompass.
A Cholecalciferol written confirmation (Cholecalciferol WC) is an official document issued by a regulatory agency to a Cholecalciferol manufacturer, verifying that the manufacturing facility of a Cholecalciferol active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Cholecalciferol APIs or Cholecalciferol finished pharmaceutical products to another nation, regulatory agencies frequently require a Cholecalciferol WC (written confirmation) as part of the regulatory process.
click here to find a list of Cholecalciferol suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Cholecalciferol as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Cholecalciferol API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Cholecalciferol as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Cholecalciferol and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Cholecalciferol NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Cholecalciferol suppliers with NDC on PharmaCompass.
Cholecalciferol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Cholecalciferol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Cholecalciferol GMP manufacturer or Cholecalciferol GMP API supplier for your needs.
A Cholecalciferol CoA (Certificate of Analysis) is a formal document that attests to Cholecalciferol's compliance with Cholecalciferol specifications and serves as a tool for batch-level quality control.
Cholecalciferol CoA mostly includes findings from lab analyses of a specific batch. For each Cholecalciferol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Cholecalciferol may be tested according to a variety of international standards, such as European Pharmacopoeia (Cholecalciferol EP), Cholecalciferol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Cholecalciferol USP).

