
Synopsis
Synopsis
0
CEP/COS
0
KDMF
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
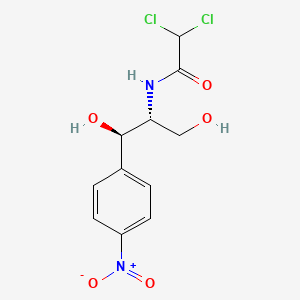

1. Amphenicol
2. Amphenicols
3. Chlornitromycin
4. Chlorocid
5. Chloromycetin
6. Cloranfenicol
7. Detreomycin
8. Kloramfenikol
9. Levomycetin
10. Ophthochlor
11. Syntomycin
1. 56-75-7
2. Chloromycetin
3. Chlornitromycin
4. Levomycetin
5. Chloroamphenicol
6. Halomycetin
7. Levomicetina
8. Chlorocid
9. Globenicol
10. Alficetyn
11. Chloramex
12. Chlorocol
13. Detreomycin
14. Oleomycetin
15. Fenicol
16. Amphenicol
17. Aquamycetin
18. Chloramficin
19. Chloramfilin
20. Chloroptic
21. Cloramicol
22. D-chloramphenicol
23. Econochlor
24. Enteromycetin
25. Juvamycetin
26. Leukomycin
27. Novomycetin
28. Ophthochlor
29. Sificetina
30. Amphicol
31. Mychel
32. Chloramphenicolum
33. Chloronitrin
34. Ciplamycetin
35. Detreomycine
36. Intramycetin
37. Laevomycetinum
38. Levomitsetin
39. Mediamycetine
40. Micochlorine
41. Novophenicol
42. Stanomycetin
43. Synthomycetin
44. Amseclor
45. Anacetin
46. Austracil
47. Austracol
48. Biocetin
49. Biophenicol
50. Chemicetin
51. Chemicetina
52. Chlomycol
53. Chloramsaar
54. Chlorasol
55. Chloricol
56. Chlorocaps
57. Chlorocide
58. Chlorovules
59. Cidocetine
60. Cloramficin
61. Cloramidina
62. Clorocyn
63. Cloromisan
64. Clorosintex
65. Comycetin
66. Cylphenicol
67. Doctamicina
68. Embacetin
69. Erbaplast
70. Farmicetina
71. Hortfenicol
72. Isicetin
73. Ismicetina
74. Isophenicol
75. Kemicetina
76. Kemicetine
77. Leukomyan
78. Loromisin
79. Mastiphen
80. Medichol
81. Micloretin
82. Micoclorina
83. Microcetina
84. Rivomycin
85. Ambofen
86. Catilan
87. Chlomin
88. Desphen
89. Emetren
90. Enicol
91. Ertilen
92. Glorous
93. Kamaver
94. Klorita
95. Isopto Fenicol
96. Chlora-tabs
97. Chlorocidin C
98. Chloroject L
99. Normimycin V
100. Chlorocid S
101. Klorocid S
102. Mychel-vet
103. Chloramfenikol
104. Cloramfenicol
105. Novochlorocap
106. Sintomicetina
107. Chloromax
108. Oftalent
109. Otachron
110. Pantovernil
111. Pentamycetin
112. Quemicetina
113. Romphenil
114. Ronphenil
115. Septicol
116. Chloro-25 Vetag
117. Mycinol
118. Opclor
119. Otophen
120. Paraxin
121. Sintomicetine R
122. Sno-phenicol
123. Chlorocidin C Tetran
124. Chlorofair
125. Cloroamfenicolo
126. Optomycin
127. D-(-)-chloramphenicol
128. Chloromycetny
129. Dextromycetin
130. Synthomycetine
131. Treomicetina
132. Tevcocin
133. Tifomycine
134. Unimycetin
135. Veticol
136. Viceton
137. Tiromycetin
138. Leukamycin
139. Loromisan
140. Tifomycin
141. D-(-)-threo-chloramphenicol
142. Tega-cetin
143. 2,2-dichloro-n-[(1r,2r)-1,3-dihydroxy-1-(4-nitrophenyl)propan-2-yl]acetamide
144. I 337a
145. U-6062
146. Nci-c55709
147. Nsc 3069
148. D-threo-chloramphenicol
149. 2,2-dichloro-n-[(1r,2r)-2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl]acetamide
150. D(-)-threo-chloramphenicol
151. D-(-)-threo-1-p-nitrophenyl-2-dichloroacetylamino-1,3-propanediol
152. Caf
153. Cloramfen
154. Ak-chlor
155. Chebi:17698
156. Nsc3069
157. Nsc-3069
158. D-(-)-2,2-dichloro-n-(beta-hydroxy-alpha-(hydroxymethyl)-p-nitrophenylethyl)acetamide
159. D-(-)-threo-1-p-nitrophenyl-2-dichloracetamido-1,3-propanediol
160. Acetamide, 2,2-dichloro-n-(2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl)-, (r-(r*,r*))-
161. Chloramphenicol (chloromycetin)
162. 125440-98-4
163. Cloramfenicolo
164. Syntomycin
165. D-(-)-threo-2-dichloroacetamido-1-p-nitrophenyl-1,3-propanediol
166. D-(-)-threo-1-(4-nitrophenyl)-2-dichloroacetamido-1,3-propanediol
167. D-threo-(1r,2r)-1-p-nitrophenyl-2-dichloroacetamido-1,3-propanediol
168. Chloramphenicol-[ring-3,5-3h]
169. Acetamide, 2,2-dichloro-n-[2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl]-, [r-(r*,r*)]-
170. 66974fr9q1
171. Ncgc00091011-05
172. Caf (pharmaceutical)
173. Dsstox_cid_265
174. Chloramfenikol [czech]
175. Chloromycetny [polish]
176. Cloramfenicolo [dcit]
177. D-threo-n-(1,1'-dihydroxy-1-p-nitrophenylisopropyl)dichloroacetamide
178. 2,2-dichloro-n-((1r,2r)-1,3-dihydroxy-1-(4-nitrophenyl)propan-2-yl)acetamide
179. Chlorbiotic (veterinary)
180. Chloramphenicol 100 Microg/ml In Ethyl Acetate
181. Cloroamfenicolo [italian]
182. Dsstox_rid_75473
183. Chloramphenicol Crystalline
184. Dsstox_gsid_20265
185. Elase-chloromycetin
186. Gloveticol
187. Mycochlorin
188. Ocuphenicol
189. Sintomicetin
190. Tyfomycine
191. Chlorocin
192. Halcetin
193. Levocin
194. Levoplast
195. Levosin
196. Levovetin
197. Myclocin
198. Soluthor
199. Chloramphenicol, D-
200. Chloroptic S.o.p.
201. Cloramfenicol [inn-spanish]
202. Chloramphenicolum [inn-latin]
203. Ophtochlor
204. Synthomycine
205. Tevcosin
206. Opelor
207. D(-)-threo-2-dichloroacetamido-1-p-nitrophenyl-1,3-propanediol
208. (-)-chloramphenicol
209. Acetamide, 2,2-dichloro-n-((1r,2r)-2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl)-
210. Econochlor (tn)
211. Amphicol (tn)
212. Ophthocort (salt/mix)
213. Acetamide, 2,2-dichloro-n-[(1r,2r)-2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl]-
214. Smr000471851
215. Chloromyxin (salt/mix)
216. Chloromycetin (tn)
217. Ccris 3922
218. Hsdb 3027
219. Sr-01000761450
220. Einecs 200-287-4
221. Elase-chloromycetin (salt/mix)
222. Brn 2225532
223. Chioramphenicol
224. Chloramphenicole
225. Ai3-25003
226. Unii-66974fr9q1
227. Cas-56-75-7
228. Ncgc00094620-01
229. Thiamphenicol,(s)
230. 2,2-dichloro-n-((1r,2r)-2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl)acetamide
231. 2787-09-9
232. D-(-)-threo-2,2-dichloro-n-(.beta.-hydroxy-.alpha.-(hydroxymethyl))-p-nitrophenethylacetamide
233. D-(-)-threo-2,2-dichloro-n-[.beta.-hydroxy-.alpha.-(hydroxymethyl)]-p-nitrophenethylacetamide
234. Chloramphenicol [usp:inn:ban:jan]
235. Chloramphenicol,(s)
236. Mfcd00078159
237. Prestwick3_000031
238. Chembl130
239. Epitope Id:114066
240. Pork Muscle-chloramphenicol
241. Chloramphenicol [mi]
242. Schembl16111
243. Bspbio_000121
244. Chloramphenicol [inn]
245. Chloramphenicol [jan]
246. Wln: Wnr Dyqy1qmvygg
247. 4-13-00-02742 (beilstein Handbook Reference)
248. Mls001055372
249. Mls001066397
250. Mls001332385
251. Mls001332386
252. Mls002222155
253. Bidd:gt0145
254. Chloramphenicol [hsdb]
255. Chloramphenicol [iarc]
256. Divk1c_000544
257. Chloramphenicol [vandf]
258. Bpbio1_000135
259. Chloramphenicol [mart.]
260. Chloramphenicolum [hpus]
261. Chloramphenicol [usp-rs]
262. Chloramphenicol [who-dd]
263. Chloramphenicol [who-ip]
264. D-(-)-threo-1-(p-nitrophenyl)-2-(dichloroacetylamino)-1,3-propanediol
265. D-(-)-threo-n-dichloroacetyl-1-p-nitrophenyl-2-amino-1,3-propanediol
266. Dtxsid7020265
267. Bdbm23447
268. Chloramphenicol, Gamma-irradiated
269. Gtpl10901
270. Hms501l06
271. Kbio1_000544
272. Ninds_000544
273. Chloramphenicol (jp17/usp/inn)
274. Hms2090m15
275. Hms2095g03
276. Hms2269n06
277. Hms3712g03
278. Zinc113382
279. Chloramphenicol, >=98% (hplc)
280. Acetamide, 2,2-dichloro-n-(.beta.-hydroxy-.alpha.-(hydroxymethyl)-p-nitrophenethyl)-, D-(-)-threo-
281. Acetamide, 2,2-dichloro-n-[.beta.-hydroxy-.alpha.-(hydroxymethyl)-p-nitrophenethyl]-, D-threo-(-)-
282. Bcp12150
283. D-(-)-threo-2,2-dichloro-n-(.beta.-hydroxy-.alpha.-(hydroxymethyl)-p-nitrophenyl-ethyl)acetamide
284. D-threo-n-dichloroacetyl-1-p-nitrophenyl-2-amino-1,3-propanediol
285. Hy-b0239
286. Rkl10087
287. Chloramphenicol [green Book]
288. Tox21_111306
289. Tox21_400061
290. Chloramphenicol [orange Book]
291. D-(-)-threo-2-dichloroacetamido-1-(4-nitrophenyl)-1,3-propanediol
292. S1677
293. Chloramphenicol [ep Monograph]
294. Akos005111001
295. Chloramphenicol For Peak Identification
296. Porcine Muscle-chloramphenicol (blank)
297. Ccg-220031
298. Chloramphenicol [usp Monograph]
299. Db00446
300. Chloramphenicolum [who-ip Latin]
301. D-threo-(-)-2,2-dichloro-n-(beta-hydroxy-alpha-(hydroxymethyl)-p-nitrophenethyl)acetamide
302. Idi1_000544
303. Smp1_000065
304. Ncgc00091011-01
305. Ncgc00091011-02
306. Ncgc00091011-03
307. Ncgc00091011-04
308. Ncgc00091011-06
309. Ncgc00091011-08
310. Ncgc00091011-09
311. Ncgc00091011-20
312. Acetamide, 2,2-dichloro-n-(2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl)-, (theta-(theta,theta))-
313. Acetamide, 2,2-dichloro-n-(beta-hydroxy-alpha-(hydroxymethyl)-p-nitrophenethyl)-, D-(-)-threo-
314. As-14683
315. Nci60_002620
316. Ophthocort Component Chloramphenicol
317. Chloramphenicol, Puriss., 98.0-102.0%
318. Chloromyxin Component Chloramphenicol
319. Ab00374860
320. Chloramphenicol 10 Microg/ml In Acetonitrile
321. Chloramphenicol, Tested According To Ph.eur.
322. Sw198497-2
323. Chloramphenicol 100 Microg/ml In Acetonitrile
324. Chloramphenicol Component Of Ophthocort
325. C-3307
326. C00918
327. Chloramphenicol Component Of Chloromyxin
328. D00104
329. Ab00374860-13
330. Ab00374860-14
331. Ab00374860_15
332. Chloramphenicol, Meets Usp Testing Specifications
333. Q274515
334. Chloramphenicol, Vetranal(tm), Analytical Standard
335. Elase-chloromycetin Component Chloramphenicol
336. Sr-01000761450-2
337. Sr-01000761450-3
338. Sr-01000761450-5
339. Brd-k08111712-001-02-7
340. Brd-k08111712-001-16-7
341. Chloramphenicol Component Of Elase-chloromycetin
342. Chloramphenicol, Antibiotic For Culture Media Use Only
343. Chloroptic-p S.o.p. Component Chloramphenicol
344. Chloramphenicol Component Of Chloroptic-p S.o.p.
345. Chloramphenicol, Bioreagent, Suitable For Plant Cell Culture
346. Chloramphenicol, Certified Reference Material, Tracecert(r)
347. Chloromycetin Hydrocortisone Component Chloramphenicol
348. Chloramphenicol Component Of Chloromycetin Hydrocortisone
349. Chloramphenicol, British Pharmacopoeia (bp) Reference Standard
350. Chloramphenicol, European Pharmacopoeia (ep) Reference Standard
351. Chloramphenicol, United States Pharmacopeia (usp) Reference Standard
352. D-threo-1-(p-nitrophenyl)-2-(dichloroacetylamino)-1,3-propanediol
353. 2,2-dichloro-n-[(1r,2r)-1,3-dihydroxy-1-(4-nitrophenyl)-2-propyl]acetamide
354. Acetamide,2,2-dichloro-n-[(1r,2r)-2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl]-
355. Acetamide,2-dichloro-n-[.beta.-hydroxy-.alpha.-(hydroxymethyl)-p-nitrophenethyl]
356. Chloramphenicol, Biotechnology Performance Certified, Suitable For Plant Cell Culture
357. D-(-)-2,2-dichloro-n-(.beta.-hydroxy-.alpha.-(hydroxymethyl)-p-nitrophenyl-ethyl)acetamide
358. 2,2-dichloro-n-(2-hydroxy-1-(hydroxymethyl)-2-(4-(hydroxy(oxido)amino)phenyl)ethyl)acetamide, (1r, 2r)-
359. Acetamide, 2,2-dichloro-n-(2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl)-, (r*,r*)-(+-)-
360. Acetamide,2-dichloro-n-[.beta.-hydroxy-.alpha.-(hydroxymethyl)-p-nitrophenethyl]-, D-threo-(-)-
361. Acetamide,2-dichloro-n-[.beta.-hydroxy-.alpha.-(hydroxymethyl)-p-nitrophenethyl]-,d-(-)-threo-
362. Acetamide,2-dichloro-n-[2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl]-, [r-(r*,r*)]-
363. Chloramphenicol 10 Microg/ml In Acetonitrile. Short Expiry Date Due To Chemical Nature Of Component(s)
364. D-(-)-threo-alpha, Alpha-dichloro-n-(beta-hydroxy-alpha-(hydroxymethyl)-p-nitrophenethyl)acetamide
365. D-threo-(-)-2,2-dichloro-n-(.beta.-hydroxy-.alpha.-(hydroxymethyl)-p-nitrophenethyl)acetamide
1. Chloramphenicol Succinate
| Molecular Weight | 323.13 g/mol |
|---|---|
| Molecular Formula | C11H12Cl2N2O5 |
| XLogP3 | 1.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Exact Mass | 322.0123269 g/mol |
| Monoisotopic Mass | 322.0123269 g/mol |
| Topological Polar Surface Area | 115 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 342 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Bacterial Agents; Protein Synthesis Inhibitors
National Library of Medicine's Medical Subject Headings. Chloramphenicol. Online file (MeSH, 2017). Available from, as of April 10, 2017: https://www.nlm.nih.gov/mesh/2017/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Chloramphenicol is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of April 10, 2017: https://clinicaltrials.gov/
Chloramphenicol is an antibiotic produced by Streptomyces venezuelae ... recommended for serious infections in which the location of the infection, susceptibility of the pathogen or poor response to other therapy indicate restricted antimicrobial option. It has been used since the 1950s for a wide range of microbial infections, including typhoid fever and other forms of salmonellosis, and central nervous system, anaerobic and ocular infections ... .
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V50 171 (1990)
(VET): Chloramphenicol Tablets are recommended for oral treatment of the following conditions in dogs: Bacterial pulmonary infections caused by susceptible microorganisms such as: Staphylococcus aureus, Streptococcus pyogenes and Brucella bronchiseptica; infections of the urinary tract caused by susceptible microorganisms such as: Escherichia coli, Proteus vulgaris, Corynebacterium renale, Streptococcus spp., and hemolytic Staphylococcus; enteritis caused by susceptible microorganisms such as: E. coli, Proteus spp., Salmonella spp., and Pseudomonas spp.; infections associated with canine distemper caused by susceptible microorganims such as: B. bronchiseptica, E. coli, P. aeruginosa, Proteus spp., Shigella spp. and Neisseria catarrhalis. Additional adjunctive therapy should be used when indicated. Most susceptible infectious disease organisms will respond to chloramphenicol therapy in three to five days when the recommended dosage regimen is followed. If no response to chloramphenicol therapy is obtained in three to five days, discontinue its use and review the diagnosis. Also, a change of therapy should be considered. Laboratory tests should be conducted including in vitro culturing and susceptibility tests on samples collected prior to treatment.
NIH; DailyMed. Current Medication Information for Viceton- chloramphenicol tablet, coated (veterinary drug) (Updated: November 2015). Available from, as of April 13, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=698aaa0a-9fd3-40c2-ec93-0dc2170a75ba&audience=consumer3
For more Therapeutic Uses (Complete) data for Chloramphenicol (38 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING Serious and fatal blood dyscrasias (aplastic anemia, hypoplastic anemia, thrombocytopenia and granulocytopenia) are known to occur after the administration of chloramphenicol. In addition, there have been reports of aplastic anemia attributed to chloramphenicol which later terminated in leukemia. Blood dyscrasias have occurred after both short-term and prolonged therapy with this drug. Chloramphenicol must not be used when less potentially dangerous agents will be effective, as described in the INDICATIONS AND USAGE section. It must not be used in the treatment of trivial infections or where it is not indicated, as in colds, influenza, infections of the throat; or as a prophylactic agent to prevent bacterial infections. Precautions: It is essential that adequate blood studies be made during treatment with the drug. While blood studies may detect early peripheral blood changes, such as leukopenia, reticulocytopenia, or granulocytopenia, before they become irreversible, such studies cannot be relied on to detect bone marrow depression prior to development of aplastic anemia. To facilitate appropriate studies and observation during therapy, it is desirable that patients be hospitalized.
NIH; DailyMed. Current Medication Information for Chloramphenicol sodium succinate injection (Updated: January 2017). Available from, as of April 12, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=aed29594-211d-49ef-813f-131975a8d0e3
/BOXED WARNING/ WARNING Bone marrow hypoplasia including aplastic anemia and death has been reported following topical application of chloramphenicol. Chloramphenicol should not be used when less potentially dangerous agents would be expected to provide effective treatment.
NIH; DailyMed. Current Medication Information for Chloromycetin Ophthalmic Ointment (Updated: October 2006). Available from, as of April 13, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=698aaa0a-9fd3-40c2-ec93-0dc2170a75ba&audience=consumer3
/Chloramphenicol/ is not recommended for the routine treatment of the typhoid carrier state.
NIH; DailyMed. Current Medication Information for Chloramphenicol sodium succinate injection (Updated: January 2017). Available from, as of April 12, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=aed29594-211d-49ef-813f-131975a8d0e3
Chloramphenicol is contraindicated in individuals with a history of previous hypersensitivity and/or toxic reaction to it. It must not be used in the treatment of trivial infections or where it is not indicated, as in colds, influenza, infections of the throat; or as a prophylactic agent to prevent bacterial infections.
NIH; DailyMed. Current Medication Information for Chloramphenicol sodium succinate injection (Updated: January 2017). Available from, as of April 12, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=aed29594-211d-49ef-813f-131975a8d0e3
For more Drug Warnings (Complete) data for Chloramphenicol (44 total), please visit the HSDB record page.
Used in treatment of cholera, as it destroys the vibrios and decreases the diarrhea. It is effective against tetracycline-resistant vibrios. It is also used in eye drops or ointment to treat bacterial conjunctivitis.
FDA Label
Chloramphenicol is a broad-spectrum antibiotic that was derived from the bacterium Streptomyces venezuelae and is now produced synthetically. Chloramphenicol is effective against a wide variety of microorganisms, but due to serious side-effects (e.g., damage to the bone marrow, including aplastic anemia) in humans, it is usually reserved for the treatment of serious and life-threatening infections (e.g., typhoid fever). Chloramphenicol is bacteriostatic but may be bactericidal in high concentrations or when used against highly susceptible organisms. Chloramphenicol stops bacterial growth by binding to the bacterial ribosome (blocking peptidyl transferase) and inhibiting protein synthesis.
Protein Synthesis Inhibitors
Compounds which inhibit the synthesis of proteins. They are usually ANTI-BACTERIAL AGENTS or toxins. Mechanism of the action of inhibition includes the interruption of peptide-chain elongation, the blocking the A site of ribosomes, the misreading of the genetic code or the prevention of the attachment of oligosaccharide side chains to glycoproteins. (See all compounds classified as Protein Synthesis Inhibitors.)
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
S01AA01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
D - Dermatologicals
D06 - Antibiotics and chemotherapeutics for dermatological use
D06A - Antibiotics for topical use
D06AX - Other antibiotics for topical use
D06AX02 - Chloramphenicol
D - Dermatologicals
D10 - Anti-acne preparations
D10A - Anti-acne preparations for topical use
D10AF - Antiinfectives for treatment of acne
D10AF03 - Chloramphenicol
G - Genito urinary system and sex hormones
G01 - Gynecological antiinfectives and antiseptics
G01A - Antiinfectives and antiseptics, excl. combinations with corticosteroids
G01AA - Antibiotics
G01AA05 - Chloramphenicol
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01B - Amphenicols
J01BA - Amphenicols
J01BA01 - Chloramphenicol
S - Sensory organs
S01 - Ophthalmologicals
S01A - Antiinfectives
S01AA - Antibiotics
S01AA01 - Chloramphenicol
S - Sensory organs
S02 - Otologicals
S02A - Antiinfectives
S02AA - Antiinfectives
S02AA01 - Chloramphenicol
S - Sensory organs
S03 - Ophthalmological and otological preparations
S03A - Antiinfectives
S03AA - Antiinfectives
S03AA08 - Chloramphenicol
Absorption
Rapidly and completely absorbed from gastrointestinal tract following oral administration (bioavailability 80%). Well absorbed following intramuscular administration (bioavailability 70%). Intraocular and some systemic absorption also occurs after topical application to the eye.
Hepatic metabolism to the inactive glucuronide is the major route of elimination. This metabolite and chloramphenicol itself are excreted in the urine following filtration and secretion.
Brunton, L. Chabner, B, Knollman, B. Goodman and Gillman's The Pharmaceutical Basis of Therapeutics, Twelth Edition, McGraw Hill Medical, New York, NY. 2011, p. 1527
Chloramphenicol administered orally is absorbed rapidly from the intestinal tract. In controlled studies in adult volunteers using the recommended dosage of 50 mg/kg/day, a dosage of 1 g every 6 hours for 8 doses was given. Using the microbiological assay method, the average peak serum level was 11.2 ug/mL one hour after the first dose. A cumulative effect gave a peak rise to 18.4 ug/mL after the fifth dose of 1 g. Mean serum levels ranged from 8 to 14 ug/mL over the 48-hour period. Total urinary excretion of chloramphenicol in these studies ranged from a low of 68% to a high of 99% over a three-day period. From 8% to 12% of the antibiotic excreted is in the form of free chloramphenicol; the remainder consists of microbiologically inactive metabolites, principally the conjugate with glucuronic acid. Since the glucuronide is excreted rapidly, most chloramphenicol detected in the blood is in the microbiologically active free form. Despite the small proportion of unchanged drug excreted in the urine, the concentration of free chloramphenicol is relatively high, amounting to several hundred mcg/mL in patients receiving divided doses of 50 mg/kg/day. Small amounts of active drug are found in bile and feces. Chloramphenicol diffuses rapidly, but its distribution is not uniform. Highest concentrations are found in liver and kidney, and lowest concentrations are found in brain and cerebrospinal fluid. Chloramphenicol enters cerebrospinal fluid even in the absence of meningeal inflammation, appearing in concentrations about half of those found in the blood. Measurable levels are also detected in pleural and in ascitic fluids, saliva, milk, and in the aqueous and vitreous humors. Transport across the placental barrier occurs with somewhat lower concentration in cord blood of neonates than in maternal blood.
NIH; DailyMed. Current Medication Information for Chloramphenicol sodium succinate injection (Updated: January 2017). Available from, as of April 12, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=aed29594-211d-49ef-813f-131975a8d0e3
Chloramphenicol achieves maximum serum levels very rapidly following oral, intravenous and intraperitoneal administration. Intramuscular injection with chloramphenicol, except certain soluble forms, results in a somewhat delayed absorption and lower serum levels than when given by the oral, intravenous, or intraperitoneal route. Chloramphenicol diffuses readily into all body tissues, but at different concentrations. Highest concentrations are found in the liver and kidney of dogs indicating that these organs are the main route of inactivation and excretion of the metabolites. The lungs, spleen, heart and skeletal muscles contain concentrations similar to that of the blood. Chloramphenicol reaches significant concentration in the aqueous and vitreous humors of the eye from the blood. A significant difference from other antibiotics is its marked ability to diffuse into the cerebrospinal fluid. Within three to four hours after administration, the concentration in the cerebrospinal fluid has reached, on the average, 50% of the concentration in the serum. If the meninges are inflamed, the percentage may be even higher. Chloramphenicol diffuses readily into milk, pleural and ascitic fluids and crosses the placenta attaining concentrations of about 75% of that of the maternal blood.
NIH; DailyMed. Current Medication Information for Viceton- chloramphenicol tablet, coated (veterinary drug) (Updated: November 2015). Available from, as of April 13, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=698aaa0a-9fd3-40c2-ec93-0dc2170a75ba&audience=consumer3
Approximately 55% of a single daily dose can be recovered from the urine of a treated dog. A small fraction of this is in the form of unchanged chloramphenicol.
NIH; DailyMed. Current Medication Information for Viceton- chloramphenicol tablet, coated (veterinary drug) (Updated: November 2015). Available from, as of April 13, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=698aaa0a-9fd3-40c2-ec93-0dc2170a75ba&audience=consumer3
For more Absorption, Distribution and Excretion (Complete) data for Chloramphenicol (21 total), please visit the HSDB record page.
Hepatic, with 90% conjugated to inactive glucuronide.
Chloramphenicol is rather rapidly metabolized, mainly in the liver, by conjugation with glucuronic acid.
NIH; DailyMed. Current Medication Information for Viceton- chloramphenicol tablet, coated (veterinary drug) (Updated: November 2015). Available from, as of April 13, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=698aaa0a-9fd3-40c2-ec93-0dc2170a75ba&audience=consumer3
Yields d-threo-2-amino-1-(p-nitrophenyl)-1,3-propanediol and chloramphenicol-beta-d-glucuronide in man. In rat. /from table/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. C-14
... /undergoes/ direct conjugation. Formation of glucuronide was shown to occur at primary rather than at secondary alcoholic group ... its major reaction of inactivation and detoxication of drug in man, and any factor which decreases its importance ... results in greatly increased toxicity. ... In newborns ... bilirubin ... acts as competitive endogenous acceptor.
Testa, B. and P. Jenner. Drug Metabolism: Chemical & Biochemical Aspects. New York: Marcel Dekker, Inc., 1976., p. 191
Chloramphenicol 3-glucuronide was the major metabolite of chloramphenicol produced by isolated rat hepatocytes although a minor metabolite was also formed.
Siliciano RF et al; Biochem Pharmacol 27 (23): 2759 (1978)
For more Metabolism/Metabolites (Complete) data for Chloramphenicol (10 total), please visit the HSDB record page.
Half-life in adults with normal hepatic and renal function is 1.5 - 3.5 hours. In patients with impaired renal function half-life is 3 - 4 hours. In patients with severely impaired hepatic function half-life is 4.6 - 11.6 hours. Half-life in children 1 month to 16 years old is 3 - 6.5 hours, while half-life in infants 1 to 2 days old is 24 hours or longer and is highly variable, especially in low birth-weight infants.
Chloramphenicol has a half-time /in humans/ ranging from 1.6 to 4.6 hr; using different techniques and in different adult patients, apparent volumes of distribution ranging from 0.2 to 3.1 l/kg have been measured ... . The half-time is considerably longer in neonates ... in 1- to 8-day-old infants the half-life ranged from 10 to over 48 hr, and in 11-day to 8-wk-old infants the range was 5-16 hr ... .
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V50 178 (1990)
The plasma half-life of chloramphenicol in adults with normal renal and hepatic function is 1.5-4.1 hours. ... The plasma half-life is 24 hours or longer in infants 1-2 days of age and approximately 10 hours in infants 10-16 days of age. The plasma half-life of chloramphenicol is prolonged in patients with markedly reduced hepatic function. In patients with impaired renal function, the plasma half-life of chloramphenicol is not significantly prolonged, although half-lives of the inactive conjugated derivatives may be prolonged.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 196
Chloramphenicol is lipid-soluble, allowing it to diffuse through the bacterial cell membrane. It then reversibly binds to the L16 protein of the 50S subunit of bacterial ribosomes, where transfer of amino acids to growing peptide chains is prevented (perhaps by suppression of peptidyl transferase activity), thus inhibiting peptide bond formation and subsequent protein synthesis.
Chloramphenicol inhibits protein synthesis in bacteria, and to a lesser extent, in eukaryotic cells. The drug readily penetrates bacterial cells, probably by facilitated diffusion. Chloramphenicol acts primarily by binding reversibly to the 50S ribosomal subunit (near the binding site for the macrolide antibiotics and clindamycin, which chloramphenicol inhibits competitively). Although binding of tRNA at the codon recognition site on the 30S ribosomal subunit is undisturbed, the drug apparently prevents the binding of the amino acid-containing end of the aminoacyl tRNA to the acceptor site on the 50S ribosomal subunit. The interaction between peptidyltransferase and its amino acid substrate cannot occur, and peptide bond formation is inhibited.
Brunton, L. Chabner, B, Knollman, B. Goodman and Gillman's The Pharmaceutical Basis of Therapeutics, Twelth Edition, McGraw Hill Medical, New York, NY. 2011, p. 1527
Chloramphenicol ... can inhibit mitochondrial protein synthesis in mammalian cells, perhaps because mitochondrial ribosomes resemble bacterial ribosomes (both are 70S) more than they do the 80S cytoplasmic ribosomes of mammalian cells. The peptidyltransferase of mitochondrial ribosomes, but not of cytoplasmic ribosomes, is inhibited by chloramphenicol. Mammalian erythropoietic cells are particularly sensitive to the drug.
Brunton, L. Chabner, B, Knollman, B. Goodman and Gillman's The Pharmaceutical Basis of Therapeutics, Twelth Edition, McGraw Hill Medical, New York, NY. 2011, p. 1527
/Chloramphenicol/ inhibits bacterial protein synthesis by interfering with the transfer of activated amino acids from soluble RNA to ribosomes. In vitro, chloramphenicol exerts mainly a bacteriostatic effect on a wide range of gram-negative and gram-positive bacteria.
NIH; DailyMed. Current Medication Information for Chloramphenicol sodium succinate injection (Updated: January 2017). Available from, as of April 12, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=aed29594-211d-49ef-813f-131975a8d0e3
/Chloramphenicol/ acts by inhibition of protein synthesis by interfering with the transfer of activated amino acids from soluble RNA to ribosomes.
NIH; DailyMed. Current Medication Information for Chloromycetin Ophthalmic Ointment (Updated: October 2006). Available from, as of April 13, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=698aaa0a-9fd3-40c2-ec93-0dc2170a75ba&audience=consumer3
For more Mechanism of Action (Complete) data for Chloramphenicol (9 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
71
PharmaCompass offers a list of Chloramphenicol Succinate Acid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Chloramphenicol Succinate Acid manufacturer or Chloramphenicol Succinate Acid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Chloramphenicol Succinate Acid manufacturer or Chloramphenicol Succinate Acid supplier.
PharmaCompass also assists you with knowing the Chloramphenicol Succinate Acid API Price utilized in the formulation of products. Chloramphenicol Succinate Acid API Price is not always fixed or binding as the Chloramphenicol Succinate Acid Price is obtained through a variety of data sources. The Chloramphenicol Succinate Acid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Chloramphenicol Succinate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Chloramphenicol Succinate, including repackagers and relabelers. The FDA regulates Chloramphenicol Succinate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Chloramphenicol Succinate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Chloramphenicol Succinate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Chloramphenicol Succinate supplier is an individual or a company that provides Chloramphenicol Succinate active pharmaceutical ingredient (API) or Chloramphenicol Succinate finished formulations upon request. The Chloramphenicol Succinate suppliers may include Chloramphenicol Succinate API manufacturers, exporters, distributors and traders.
click here to find a list of Chloramphenicol Succinate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Chloramphenicol Succinate DMF (Drug Master File) is a document detailing the whole manufacturing process of Chloramphenicol Succinate active pharmaceutical ingredient (API) in detail. Different forms of Chloramphenicol Succinate DMFs exist exist since differing nations have different regulations, such as Chloramphenicol Succinate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Chloramphenicol Succinate DMF submitted to regulatory agencies in the US is known as a USDMF. Chloramphenicol Succinate USDMF includes data on Chloramphenicol Succinate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Chloramphenicol Succinate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Chloramphenicol Succinate suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Chloramphenicol Succinate Drug Master File in Japan (Chloramphenicol Succinate JDMF) empowers Chloramphenicol Succinate API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Chloramphenicol Succinate JDMF during the approval evaluation for pharmaceutical products. At the time of Chloramphenicol Succinate JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Chloramphenicol Succinate suppliers with JDMF on PharmaCompass.
A Chloramphenicol Succinate written confirmation (Chloramphenicol Succinate WC) is an official document issued by a regulatory agency to a Chloramphenicol Succinate manufacturer, verifying that the manufacturing facility of a Chloramphenicol Succinate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Chloramphenicol Succinate APIs or Chloramphenicol Succinate finished pharmaceutical products to another nation, regulatory agencies frequently require a Chloramphenicol Succinate WC (written confirmation) as part of the regulatory process.
click here to find a list of Chloramphenicol Succinate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Chloramphenicol Succinate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Chloramphenicol Succinate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Chloramphenicol Succinate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Chloramphenicol Succinate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Chloramphenicol Succinate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Chloramphenicol Succinate suppliers with NDC on PharmaCompass.
Chloramphenicol Succinate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Chloramphenicol Succinate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Chloramphenicol Succinate GMP manufacturer or Chloramphenicol Succinate GMP API supplier for your needs.
A Chloramphenicol Succinate CoA (Certificate of Analysis) is a formal document that attests to Chloramphenicol Succinate's compliance with Chloramphenicol Succinate specifications and serves as a tool for batch-level quality control.
Chloramphenicol Succinate CoA mostly includes findings from lab analyses of a specific batch. For each Chloramphenicol Succinate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Chloramphenicol Succinate may be tested according to a variety of international standards, such as European Pharmacopoeia (Chloramphenicol Succinate EP), Chloramphenicol Succinate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Chloramphenicol Succinate USP).

