



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0

USA (Orange Book)
0

Europe

Canada

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
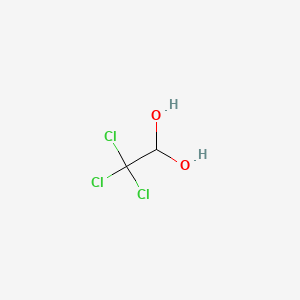

1. Hydrate, Chloral
2. Noctec
1. 302-17-0
2. 2,2,2-trichloroethane-1,1-diol
3. Trichloroacetaldehyde Hydrate
4. Noctec
5. Tosyl
6. Chloral Monohydrate
7. Chloraldurat
8. Phaldrone
9. Nycoton
10. Chloradorm
11. Chloralhydrate
12. Kessodrate
13. 1,1-ethanediol, 2,2,2-trichloro-
14. Cohidrate
15. Felsules
16. Lorinal
17. Oradrate
18. Rectules
19. Trawotox
20. Dormal
21. Escre
22. Hynos
23. Nycton
24. Somnos
25. Sontec
26. Knockout Drops
27. Somni Sed
28. Sk-chloral Hydrate
29. Aquachloral
30. Somnote
31. Trichloracetaldehyd-hydrat
32. Chlorali Hydras
33. 2,2,2-trichloro-1,1-ethanediol
34. Hydrate De Chloral
35. 1,1,1-trichloro-2,2-ethanediol
36. Trichloroacetaldehyde Monohydrate
37. Chloralhydrat
38. Hydral
39. 1,1,1-trichloro-2,2-dihydroxyethane
40. Bi 3411
41. Chloralum
42. Lycoral
43. Cloral Hydrate
44. Nsc 3210
45. Nsc-3210
46. Dtxsid7020261
47. Chebi:28142
48. 418m5916wg
49. Ncgc00159374-02
50. Ncgc00159374-03
51. Kloralhydrat
52. Chloralex
53. Chloralvan
54. Novochlorhydrate
55. Dsstox_cid_261
56. Dsstox_rid_75470
57. Dsstox_gsid_20261
58. Chloraldural [swiss]
59. Caswell No. 168
60. Chloraldurat [german]
61. Chloraldural
62. Nortec
63. Cas-302-17-0
64. Trichloroacetaldehyde, Hydrated
65. Ccris 4142
66. Hsdb 222
67. Aquachloral Supprettes
68. Trichloracetaldehyd-hydrat [german]
69. Einecs 206-117-5
70. Epa Pesticide Chemical Code 268100
71. Brn 1698497
72. Ai3-00082
73. Dea No. 2465
74. Ethanediol, 2,2,2-trichloro-
75. Unii-418m5916wg
76. Chloral Hydrate [usp:ban:jan]
77. Mfcd00044479
78. Noctec (tn)
79. 201612-49-9
80. Trichloroethanal Hydrate
81. Wln: Qyqxggg
82. Chloralum [hpus]
83. Chloral-[13c] Hydrate
84. 1, 2,2,2-trichloro-
85. Schembl34327
86. Chloral Hydrate [mi]
87. 4-01-00-03143 (beilstein Handbook Reference)
88. Chloral Hydrate [jan]
89. Chloral Hydrate (jp17/usp)
90. Chloral Hydrate [hsdb]
91. Chloral Hydrate [iarc]
92. Chembl455917
93. Chloral Hydrate [vandf]
94. Cloral Hydrate [mart.]
95. 2,2-trichloro-1,1-ethanediol
96. Chloral Hydrate [who-dd]
97. Chloral Hydrate [who-ip]
98. Nsc3210
99. Chloral Hydrate, P.a., 99.0%
100. Bcp31225
101. Zinc3872049
102. 1,1-trichloro-2,2-dihydroxyethane
103. Tox21_111614
104. Tox21_200110
105. Chloral Hydrate [green Book]
106. Stl445706
107. Chloral Hydrate [ep Impurity]
108. Akos009157238
109. Chloral Hydrate [ep Monograph]
110. Tox21_111614_1
111. Chloral Hydrate [usp Monograph]
112. Chlorali Hydras [who-ip Latin]
113. Db01563
114. Ncgc00159374-04
115. Ncgc00257664-01
116. Chloral Hydrate;2,2,2-trichloro-1,1-ethanediol;1,1-ethanediol, 2,2,2-trichloro-;choral Hydrate;2,2,2-trichloroethane-1,1-diol;2,2,2trichloroethane1,1diol
117. Ls-12935
118. Db-047727
119. Chloral Hydrate 1000 Microg/ml In Methanol
120. Chloral Hydrate, Saj First Grade, >=99.5%
121. Trichloro Acetaldehyde Hydrate Chloral Hydrate
122. C06899
123. Chloral Hydrate, Crystallized, >=98.0% (t)
124. Chloral Hydrate, Saj Special Grade, >=99.7%
125. Chloralhydrate 1000 Microg/ml In Acetonitrile
126. D00265
127. A936505
128. Q412340
129. J-520014
130. Q-200826
131. F0001-0929
132. 2,2,2-trichloroethane-1,1-diol;trichloroacetaldehyde Hydrate
133. Chloral Hydrate Solution, 1000 Mug/ml In Acetonitrile, Analytical Standard
134. Chloral Hydrate, Meets Analytical Specification Of Ph. Eur., Bp, Usp, 99.5-101%
| Molecular Weight | 165.40 g/mol |
|---|---|
| Molecular Formula | C2H3Cl3O2 |
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 163.919862 g/mol |
| Monoisotopic Mass | 163.919862 g/mol |
| Topological Polar Surface Area | 40.5 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 56.4 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Sedatives, Nonbarbiturate; Anesthetics, Intravenous
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Chloral hydrate also has been used as an adjunct to opiates and analgesics in postoperative care and control of pain. However, it generally has been replaced by agents with better pharmacokinetic and pharmacodynamic profiles. /Included in US product labeling/
USP. Convention. USPDI - Drug Information for the Health Care Professional. 20th ed. Volume I. Micromedex, Inc. Englewood, CO., 2000. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 859
Chloral hydrate has been used as a routine sedative. However, it generally has been replaced by safer and more effective agents. /Included in US product labeling/
USP. Convention. USPDI - Drug Information for the Health Care Professional. 20th ed. Volume I. Micromedex, Inc. Englewood, CO., 2000. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 859
Chloral hydrate has been used for the treatment of insomnia. However, this medication is effective as a hypnotic only for short-term use; it has been shown to lose its effectiveness for both inducing and maintaining sleep after 2 weeks of administration. In addition, chloral hydrate generally has been replaced by agents with better pharmacokinetic and pharmacodynamic profiles. /Included in US product labeling/
USP. Convention. USPDI - Drug Information for the Health Care Professional. 20th ed. Volume I. Micromedex, Inc. Englewood, CO., 2000. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 859
For more Therapeutic Uses (Complete) data for CHLORAL HYDRATE (13 total), please visit the HSDB record page.
... /Chloral hydrate/ crosses the placenta and the effects of the drug on the fetus are unknown. The manufactures caution that the use of chloral hydrate durring pregnancy may cause withdrawal symptoms in neonates
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 2610
Prolonged use of chloral hydrate may produce tolerance and physical and/or psychologic dependence. Tolerance and psychological dependence may develop by the second week of continued therapy.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 2610
Cutaneous reactions to chloral hydrate are not common but have included scarlatiniform or erythematous rash, urticaria, angioedema, purpura, eczema, bullous lesions, and erythema multiforme. Sometimes cutaneous reactions have been accompanied by fever.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 2609
Gastric irritation manifested by nausea, vomiting, and diarrhea is the most frequent adverse effect of oral chloral hydrate administration. Gastric irritation may be minimized by diluting the oral solution with water or other liquid or administering other oral dosage forms with liquids. Flatulence and unpleasant taste may also occur.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 2609
For more Drug Warnings (Complete) data for CHLORAL HYDRATE (14 total), please visit the HSDB record page.
The lethal oral dose of chloral hydrate in adults is about 10 g; however, ingestion of 4 g has caused death, and some patients have survived ingestion of as much as 30 g.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 2610
Lethal doses /of chloral hydrate/ are between 5 and 10 g.
Gossel, T.A., J.D. Bricker. Principles of Clinical Toxicology. 3rd ed. New York, NY: Raven Press, Ltd., 1994., p. 315
Mainly used as a hypnotic in the treatment of insomnia; however, it is only effective as a hypnotic for short-term use. May be used as a routine sedative preoperatively to decrease anxiety and cause sedation and/or sleep with respiration depression or cough reflex.
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)
N - Nervous system
N05 - Psycholeptics
N05C - Hypnotics and sedatives
N05CC - Aldehydes and derivatives
N05CC01 - Chloral hydrate
Absorption
Rapidly absorbed in the GI tract following oral or rectal administration. Chloral hydrate and its active metabolite, trichloroethanol, have been detected in CSF, umbilical cord blood, fetal blood, and amniotic fluid.
Route of Elimination
Trichloroethanol, trichloroethanol glucuronide, and trichloroacetic acid are excreted in the urine. Some trichloroethanol glucuronide may be secreted into bile and excreted in the feces.
Following therapeutic doses of chloral hydrate, only small, clinically insignificant amounts of the active metabolite are distributed into milk.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 2610
Chloral hydrate is rapidly absorbed from the GI tract following oral or rectal administration. Plasma concentrations of chloral hydrate (or the major metabolite, trichloroethanol) required for sedative or hypnotic effects are unknown. Following administration of a single chloral hydrate dose of 15 mg/kg, peak plasma concentrations of trichloroethanol ranged from 7-10 ug/mL in one study.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 2610
After oral administration, chloral hydrate is rapidly absorbed from the gastrointestinal tract. Peak levels of trichloroethanol and trichloroethanol glucuronide were reached within 20- 60 min after oral administration of aqueous solutions.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V63 251 (1995)
The volume of distribution of chloral hydrate is 0.6 L/kg.
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 586
For more Absorption, Distribution and Excretion (Complete) data for CHLORAL HYDRATE (11 total), please visit the HSDB record page.
Metabolized by the liver and erythrocytes to form trichloroethanol, an active metabolite. This reaction is catalyzed by alcohol dehydrogenase and other enzymes. Oxidation of chloral hydrate and trichloroethanol to trichloroacetic acid in the liver and kidneys also occurs to a lesser extent. Trichloroethanol also undergoes glucuronidation to produce an inactive metabolism.
/Chloral hydrate/ biotransformation to trichloroethanol must be rapid, since no parent compound could be detected in even the first samples taken 10 min after administration of 15 mg/kg bw to volunteers.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V63 251 (1995)
Chloral hydrate is metabolized by the liver and erythrocytes to form trichloroethanol (an active metabolite). The reduction of chloral hydrate to trichloroethanol (the major metabolite) is catalyzed by alcohol dehydrogenase and other enzymes. ... A small but variable amount of chloral hydrate and a larger portion of trichloroethanol are oxidized to trichloroacetic acid (an inactive metabolite), mainly in the liver and kidneys. Trichloroethanol may also be conjugated with glucuronic acid to form trichloroethanol glucuronide (urochloralic acid), an inactive metabolite. ... The quantities of metabolites excreted in the urine appear to be quite variable not only between different individuals but may even vary in the same individual on different days.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 2610
In mammalian species, chloral hydrate is rapidly reduced to trichloroethanol, the metabolite that appears to be responsible for the hypnotic properties of the drug. ... In rodents, a slightly different metabolic pattern is seen, as chloral hydrate is oxidized directly to trichloroacetic acid, and the oxidative pathway from trichloroethanol to trichloroacetate that is observed in humans seems to be absent.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V63 251 (1995)
As < 50% of an administered dose of chloral hydrate was recovered as metabolites in urine, yet unknown biotransformation reactions may exist for chloral hydrate in humans.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V63 251 (1995)
For more Metabolism/Metabolites (Complete) data for CHLORAL HYDRATE (10 total), please visit the HSDB record page.
Chloral hydrate is a known human metabolite of Trichloroethylene.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The plasma half-life of trichloroethanol, the active metabolite, is about 7 to 10 hours.
USP. Convention. USPDI - Drug Information for the Health Care Professional. 20th ed. Volume I. Micromedex, Inc. Englewood, CO., 2000. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 859
The average half-life of trichloroethanol glucuronide was 6.7 hr. The average plasma half-life for chloral hydrate metabolites was 8.2 hr; the half-life of the third chloral hydrate metabolite, trichloroacetic acid, was about four days, as it binds extensively to plasma proteins.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V63 251 (1995)
The plasma half-life for therapeutic doses of chloral hydrate is 4 to 5 min, whereas for trichloroethanol /a metabolite/ is 8 to 12 hr and for trichloroacetic acid /a metabolite/, 67 hr.
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 586
This study was designed to characterize the kinetics of chloral hydrate (CH) metabolism, and the formation and elimination of trichloroacetate (TCA), dichloroacetate (DCA), trichloroethanol (TCOH), and trichloroethanol glucuronide (TCOG) in male B6C3F1 mice. Mice were dosed with 67.8, 678, and 2034 umol/kg of CH through the tail vein. ... After intravenous administration, CH rapidly disappeared from blood with a terminal half-life ranging from 5 to 24 min. ... The terminal half-lives of TCOH and TCOG were similar, ranging from 0.2 to 0.7 hr. ...
PMID:8971140 Abbas R et al; Drug Metab Dispos 24 (12): 1340-6 (1996)
Chloral hydrate has CNS depressant effects similar to those of paraldehyde and the barbiturates. The mechanism of action of the drug is not completely known. The CNS depressant effect of chloral hydrate is believed to result mainly from its metabolite, trichloroethanol, although some animal studies have indicated that the rapid onset of sedation and hypnosis that chloral hydrate produces may be due to chloral hydrate itself and that the prolonged duration of action may be due to trichloroethanol.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 2610


