
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
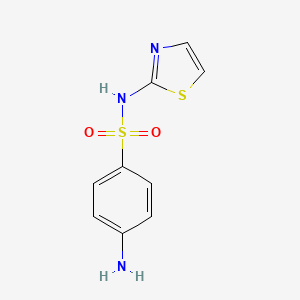

1. 4-amino-n-2-thiazolyl Benzenesulfonamide
2. Alphadol
3. Benzenesulfonamide, 4 Amino N 2 Thiazolyl
4. Benzenesulfonamide, 4-amino-n-2-thiazolyl
5. N(1)-2-thiazolylsulfanilamide
6. Norsulfazole
7. Sulfathiazole Sodium
8. Sulfathiazole, Monosodium Salt
1. 72-14-0
2. Sulphathiazole
3. Sulfathiazol
4. 2-sulfanilamidothiazole
5. Sulfanilamidothiazole
6. Thiazamide
7. Norsulfazole
8. 4-amino-n-(thiazol-2-yl)benzenesulfonamide
9. 2-sulfonamidothiazole
10. Norsulfasol
11. 2-(sulfanilylamino)thiazole
12. Neostrepsan
13. Sulfocerol
14. Thiozamide
15. Sulzol
16. 2-sulfanilamidothiazol
17. 2-(p-aminobenzenesulfonamido)thiazole
18. Azoquimiol
19. Azoseptale
20. Norsulfazol
21. Poliseptil
22. Sanotiazol
23. Sulfathiazolum
24. Sulfatiazol
25. Thiacoccine
26. Thiasulfol
27. Wintrazole
28. Cerazole
29. Chemosept
30. Cibazol
31. Eleudron
32. Estafilol
33. Planomide
34. Septozol
35. Duatok
36. Dulana
37. N(1)-2-thiazolylsulfanilamide
38. Coco-thiazole
39. Formosulfathiazole
40. Streptosilthiazole
41. 4-amino-n-2-thiazolylbenzenesulfonamide
42. Sulfamul
43. 2-(p-aminobenzenesulphonamido)thiazole
44. Usaf Sn-9
45. Cerazol (suspension)
46. Ciba 3714
47. 4-amino-n-(1,3-thiazol-2-yl)benzenesulfonamide
48. N1-(2-thiazolyl)sulfanilamide
49. Benzenesulfonamide, 4-amino-n-2-thiazolyl-
50. 4-amino-n-(1,3-thiazol-2-yl)benzene-1-sulfonamide
51. N1-2-thiazolylsulfanilamide
52. 4-amino-n-1,3-thiazol-2-ylbenzenesulfonamide
53. M&b 760
54. Rp 2090
55. Sulfanilamide, N1-2-thiazolyl-
56. 4-amino-n-thiazol-2-yl-benzenesulfonamide
57. 2090 R.p.
58. N(sup 1)-2-thiazolylsulfanilamide
59. M+b 760
60. Chebi:9337
61. Y7fks2xwqh
62. Nsc-31812
63. Chembl437
64. Nsc-683531
65. Triple Sulfa (sulfathiazole)
66. N'-(2-thiazolyl)sulfanilamide
67. N(sup1)-(2-thiazolyl)sulfanilamide
68. Nsc683531
69. Sulfanilamide, N(sup1)-2-thiazolyl-
70. Cas-72-14-0
71. Ncgc00016309-02
72. Ncgc00016309-06
73. Norsulfazolum
74. Sulfathiazole 100 Microg/ml In Acetonitrile
75. Dsstox_cid_6068
76. Dsstox_rid_78004
77. Dsstox_gsid_26068
78. Solfatiazolo [dcit]
79. Caswell No. 809b
80. Solfatiazolo
81. Sulfathiazol [inn-french]
82. Sulfatiazol [inn-spanish]
83. Sulfathiazolum [inn-latin]
84. Ccris 765
85. 2090 Rp
86. 2-sulfanilamidothiazol [german]
87. Hsdb 4380
88. Sr-05000001722
89. Unii-y7fks2xwqh
90. Sulfanilamide, N(1)-2-thiazolyl-
91. Einecs 200-771-5
92. Nsc 31812
93. Epa Pesticide Chemical Code 077903
94. Nsc 683531
95. Sulfanilamide, N(sup 1)-2-thiazolyl-
96. Sulfthiazole
97. Enterobiocine
98. Sulfavitina
99. Cerazol
100. Ai3-01050
101. Sulfathiazole [usp:inn:ban]
102. 2-sulfathiazole
103. Ytz
104. Prestwick_430
105. Mfcd00005319
106. Sulfathiazole-13c6
107. Spectrum_001000
108. 4-amino-n-(2-thiazolyl)benzenesulfonamide
109. Prestwick0_000016
110. Prestwick1_000016
111. Prestwick2_000016
112. Prestwick3_000016
113. Spectrum2_000841
114. Spectrum3_001729
115. Spectrum4_000348
116. Spectrum5_001441
117. Sulfathiazole (usp/inn)
118. N-2-thiazolylsulfanilamide
119. Sulfathiazole [mi]
120. Epitope Id:122234
121. Sulfathiazole [inn]
122. Cambridge Id 5251400
123. Cid_5340
124. Sulfathiazole [hsdb]
125. Oprea1_105970
126. Oprea1_297844
127. Schembl94165
128. Bspbio_000051
129. Bspbio_003378
130. Kbiogr_000755
131. Kbioss_001480
132. Sulfathiazole [vandf]
133. [(4-aminophenyl)sulfonyl]-1,3-thiazol-2-ylamine
134. Mls002154174
135. N-1-2-thiazolylsulfanilamide
136. Divk1c_000560
137. Spectrum1500553
138. Sulfathiazole [mart.]
139. Sulfathiazole-d4(benzene-d4)
140. Spbio_000821
141. Spbio_001972
142. Sulfathiazole [usp-rs]
143. Sulfathiazole [who-dd]
144. Bpbio1_000057
145. Wln: T5n Csj Bmswr Dz
146. Dtxsid8026068
147. Hms501l22
148. Kbio1_000560
149. Kbio2_001480
150. Kbio2_004048
151. Kbio2_006616
152. Kbio3_002598
153. N(sup1)-2-thiazolylsulfanilamide
154. Ninds_000560
155. Hms1568c13
156. Hms1921c07
157. Hms2092k09
158. Hms2095c13
159. Hms2259a13
160. Hms3652a03
161. Hms3712c13
162. Pharmakon1600-01500553
163. Sulfathiazole [green Book]
164. Zinc121458
165. Sulfathiazole [orange Book]
166. Amy33440
167. Hy-b0507
168. Nsc31812
169. Sulfathiazole (triple Sulfa)
170. Sulfathiazole [ep Monograph]
171. Tox21_110363
172. Tox21_202243
173. Tox21_303238
174. Bdbm50027796
175. Ccg-40296
176. Nsc757331
177. S3116
178. Stk043870
179. Sulfathiazole [usp Monograph]
180. Trysul Component Sulfathiazole
181. 2-(4-aminobenzenesulfonamido)thiazole
182. Sultrin Component Sulfathiazole
183. Vagilia Component Sulfathiazole
184. Akos000108630
185. Tox21_110363_1
186. Db06147
187. Nsc-757331
188. Sdccgmls-0065585.p001
189. Gyne-sulf Component Sulfathiazole
190. Idi1_000560
191. Sulfathiazole Component Of Trysul
192. Ncgc00016309-01
193. Ncgc00016309-03
194. Ncgc00016309-04
195. Ncgc00016309-05
196. Ncgc00016309-07
197. Ncgc00016309-08
198. Ncgc00016309-09
199. Ncgc00016309-10
200. Ncgc00016309-14
201. Ncgc00091133-01
202. Ncgc00091133-02
203. Ncgc00091133-03
204. Ncgc00091133-04
205. Ncgc00257187-01
206. Ncgc00259792-01
207. Sulfathiazole 100 Microg/ml In Methanol
208. Sulfathiazole Component Of Sultrin
209. Sulfathiazole Component Of Vagilia
210. Ac-12783
211. Ds-17245
212. Nci60_002730
213. Smr000017368
214. Pyridine,2-(chloromethyl)-3,4-dimethoxy-
215. Sbi-0051527.p004
216. Sulfanilamide, N1-4-thiazolin-2-ylidene-
217. Db-055610
218. Sulfathiazole Component Of Gyne-sulf
219. 4-amino-n-(thiazol-2-yl)-benzenesulfonamide
220. Ab00052102
221. Bb 0245015
222. Ft-0631310
223. Sw149625-4
224. 4-[(1,3-thiazol-2-yl)aminosulfonyl]aniline
225. Sulfathiazole, Analytical Standard, >=98.0%
226. D01047
227. D70411
228. Ab00052102_14
229. Ab00052102_15
230. Q408427
231. Sulfathiazole, Vetranal(tm), Analytical Standard
232. Triple Sulfa (sulfathiazole) [orange Book]
233. 4-amino-n-(1,3-thiazol-2-yl)benzenesulfonamide #
234. Q-201765
235. Sr-05000001722-1
236. Sr-05000001722-3
237. Sulfathiazole, Antibiotic For Culture Media Use Only
238. Brd-k14705039-001-05-7
239. Brd-k14705039-001-08-1
240. F1443-4816
241. Sulfathiazole, European Pharmacopoeia (ep) Reference Standard
242. Sulfathiazole, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 255.3 g/mol |
|---|---|
| Molecular Formula | C9H9N3O2S2 |
| XLogP3 | 0.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 255.01361889 g/mol |
| Monoisotopic Mass | 255.01361889 g/mol |
| Topological Polar Surface Area | 122 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 320 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Infective Agents /SRP: Antibacterial/
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
The US FDA announced on May 31, 1979, that its Anti-infective and Topical Drugs Advisory Committee and Fertility and Maternal health Advisory Committee, as well as other studies, had concluded there was no adequate evidence that the then-available vaginal sulfonamides formulations were effective either for the treatment of vulvovaginitis caused by Candida albicans, trichomonas vaginalis, or Gardnerella vaginalis (Hemophilus vaginalis) or for relief of the symptoms of these conditions. /Sulfonamides/
USP. Convention. USPDI - Drug Information for the Health Care Professional. 20th ed. Volume I. Micromedex, Inc. Englewood, CO., 2000. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2857
In the opinion of USP medical experts, triple sulfa vaginal preparations are not effective for any indication, including vulvovaginitis caused by Gardnerella vaginalis and use as a deodorant in saprophytic infections following radiation therapy. Also, USP medical experts do not recommend the use of vaginal sulfonamides, including the reformulated single-entry preparations, for the treatment of fungal infections of the vagina. /Sulfonamides/
USP. Convention. USPDI - Drug Information for the Health Care Professional. 20th ed. Volume I. Micromedex, Inc. Englewood, CO., 2000. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2857
MEDICATION (VET): Antibacterial
Budavari, S. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 1996., p. 1529
For more Therapeutic Uses (Complete) data for SULFATHIAZOLE (8 total), please visit the HSDB record page.
The number of conditions for which the sulfonamides are therapeutically useful and constitute drugs of first choice has been reduced sharply by the development of more effective antimicrobial agents and by the gradual increase in the resistance of a number of bacterial species to this class of drugs. /Sulfonamides/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1062
Many of the adverse effects that have been attributed to the sulfonamides appear to be hypersensitivity reactions. The incidence of hypersensitivity reactions appears to increase with increased sulfonamide dosage. Although cross-sensitization has been reported to occur between the various anti-infective sulfonamides, some diuretics such as acetazolamide and the thiazides, some goitrogens, and sulfonylurea antidiabetic agents, the association between hypersensitivity to sulfonamide anti-infectives and subsequent sensitivity reactions to non-anti-infective sulfonamides (e.g., thiazides, sulfonylurea antidiabetic agents, furosemide, dapsone, probenecid) appears to result from a predisposition to allergic reactions in general rather than to cross-sensitivity to the sulfa moiety per se. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 423
Various dermatologic reactions, including rash, pruritus, urticaria, erythema nodosum, erythema multiforme (Stevens-Johnson syndrome), Lyell's syndrome (may be associated with corneal damage), Behcet's syndrome, toxic epidermal necrolysis, and exfoliative dermatitis, have been reported in patients receiving sulfonamides. Because photosensitivity may also occur, patients should be cautioned against exposure to UV light or prolonged exposure to sunlight. A relatively high proportion of fatalities has occurred as a result of the Stevens-Johnson syndrome, especially in children. Although long-acting sulfonamides (which are no longer commercially available) have been associated most often with the Stevens-Johnson syndrome, other sulfonamides also have been reported to cause this reaction. The physician should be alert to the signs, including high fever, severe headache, stomatitis, conjunctivitis, rhinitis, urethritis, and balanitis, which may precede the onset of the cutaneous lesions of the Stevens-Johnson syndrome. If a rash develops during therapy, the sulfonamide should be discontinued at once. In rare instances, a skin rash may precede a more serious reaction such as Stevens-Johnson syndrome, toxic epidermal necrolysis, hepatic necrosis, and/or serious blood disorders. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 423
Fever, which may develop 7-10 days after the initial sulfonamide dose, is a common adverse effect of sulfonamide therapy. Serum sickness syndrome or serum sickness-like reactions (e.g., fever, chills, rigors, flushing, joint pain, urticarial eruptions, conjunctivitis, bronchospasm, leukopenia), have been reported; rarely, anaphylactoid reactions and anaphylaxis may occur. Lupus erythematosus-like syndrome, disseminated lupus erythematosus, angioedema, vasculitis, vascular lesions including periarteritis nodosa and arteritis, cough, shortness of breath, chills, pulmonary infiltrates, pneumonitis (which may be associated with eosinophilia), fibrosing alveolitis, pleuritis, pericarditis with or without tamponade, allergic myocarditis, hepatitis, hepatic necrosis with or without immune complexes, parapsoriasis varioliformis acuta, alopecia, conjunctival and scleral injection, periorbital edema, and arthralgia have also been reported. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 423
For more Drug Warnings (Complete) data for SULFATHIAZOLE (23 total), please visit the HSDB record page.
Sulfathiazole is effective against a wide range of gram positive and gram negative pathogenic microorganisms. Although no longer used in humans, it is used in cattle.
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
D06BA02
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
D06BA02
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
D06BA02
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
D06BA02
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
D - Dermatologicals
D06 - Antibiotics and chemotherapeutics for dermatological use
D06B - Chemotherapeutics for topical use
D06BA - Sulfonamides
D06BA02 - Sulfathiazole
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01E - Sulfonamides and trimethoprim
J01EB - Short-acting sulfonamides
J01EB07 - Sulfathiazole
Individual sulfonamides differ markedly in their absorption, distribution, and elimination. With the exception of sulfapyrimidine and sulfasalazine, which are only slightly absorbed, sulfonamides are generally well absorbed from the GI tract. Approximately 70-90% of an oral dose of the absorbable sulfonamides is reportedly absorbed from the small intestine; small amounts may also be absorbed from the stomach. Sulfamethizole and sulfisoxazole (no longer commercially available in the US) are absorbed rapidly; peak blood concentrations are usually obtained within 2-4 hours. Sulfadiazine and sulfapyridine are absorbed at a slower rate with peak blood concentrations occurring within 3-7 hours. Administration of oral sulfonamides with food appears to delay, but not reduce, absorption of the drugs. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 425
Absorption of sulfonamides from the vagina, respiratory tract, or abraded skin is variable and unreliable; however, enough drug may be absorbed to induce sensitization or toxicity. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 425
Although only free (unmetabolized and unbound) sulfonamides are microbiologically active, blood concentrations are often determined on the basis of total sulfonamide concentration. Generally, sulfonamide plasma concentrations are approximately twice the blood concentrations. Wide variations in blood concentrations have been reported in different individuals receiving identical doses of the same sulfonamide. Blood total sulfonamide concentrations of 12-15 mg/dL have been reported to be optimal; blood concentrations greater than 20 mg/dL have been associated with an increased incidence of adverse reactions. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 425
Absorbable sulfonamides are widely distributed in the body. Although most sulfonamides appear to cross cell membranes, sulfisoxazole appears to be distributed only in extracellular fluid. Sulfonamides may appear in pleural, peritoneal, synovial, amniotic, prostatic, and seminal vesicular fluid, and aqueous humor. Concentrations of some sulfonamides in the CSF may reach 35-80% of blood concentrations. Small amounts of sulfonamides are also distributed into sweat, tears, saliva, and bile. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 425
For more Absorption, Distribution and Excretion (Complete) data for SULFATHIAZOLE (16 total), please visit the HSDB record page.
Metabolism of sulfonamide drugs in animals includes conjugation at the N4-position (acetyl, sulfate, glucuronic acid, and glucose), conjugation at the N1-position (sulfate and glucuronic acid), removal of the p-amino group (formation of the desamino metabolite), ring hydroxylation, and conjugation of the ring hydroxylation products. Dietary nitrite enhances the production of the desamino metabolite of sulfathiazole. The intermediate leading to the desamino metabolite of sulfamethazine is weakly mutagenic in the Ames test (Nelson et al., 1987; Paulson et al., 1987).
Although the liver is the major site of metabolism, sulfonamides may also be metabolized in other body tissues. Most sulfonamides are metabolized mainly by N4-acetylation. The degree of acetylation, which is a function of time, varies from less than 5% for sulfamethizole to up to 40% for sulfadiazine. The N4-acetyl metabolites, which do not possess antibacterial activity, have greater affinity for plasma albumin than does the nonacetylated drug and are usually less soluble than the parent sulfonamide, particularly in acidic urine. Like acetyl derivatives, glucuronide derivatives do not possess antibacterial activity; however, glucuronide derivatives are water soluble, appear to resemble the nonacetylated sulfonamide in plasma binding capacity, and have not been associated with adverse effects. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 425
Metabolism of sulfonamide drugs in animals includes conjugation at the N4-position (acetyl, sulfate, glucuronic acid, and glucose), conjugation at the N1-position (sulfate and glucuronic acid), removal of the p-amino group (formation of the desamino metabolite), ring hydroxylation, and conjugation of the ring hydroxylation products. Dietary nitrite enhances the production of the desamino metabolite of sulfathiazole.
WHO/FAO; Joint Expert Committee on Food Additives; Food Additive Series 25: Sulfathiazloe (72-14-0) (1990). Available from, as of July 21, 2008: https://www.inchem.org/pages/jecfa.html
Sulfathiazole...is one of the short-acting sulfonamides & in man is excreted in urine as unchanged sulfathiazole (63% of dose), N4-acetylsulfathiazole (29%), sulfathiazole-N4-glucuronide (0.8%), sulfathiazole-N4-sulfate (0.5%) & sulfathiazole-N1-glucuronide (3.8%).
Parke, D. V. The Biochemistry of Foreign Compounds. Oxford: Pergamon Press, 1968., p. 180
Deposition kinetics, metabolism and urinary excretion of sulfathiazole were investigated in German black head sheep following single oral administration (100 mg/kg). Kinetic evaluation of plasma levels was performed using a two-compartment best fit model. Sulfathiazole is significantly metabolized to N4-acetyl metabolite in the rumen fluid. The drug is very poorly absorbed since the minimum effective concentration in plasma was not attained at any time following oral administration. The prolonged elimination half-life in sheep may be due to a low rate of drug absorption from the rumen and gastro-intestinal tract. Sulfathiazole was mainly excreted in the urine as free drug and N4-acetyl metabolite.
PMID:8591739 Jain S, Hapke H; Dtsch Tierarztl Wochenschr 102 (10): 394-5 (1995)
For more Metabolism/Metabolites (Complete) data for SULFATHIAZOLE (6 total), please visit the HSDB record page.
Sulfonamides are generally classified as short-acting, intermediate-acting, or long-acting depending on the rate at which they are absorbed and eliminated. Sulfamethizole, sulfasalazine, and sulfisoxazole are generally considered to be short-acting sulfonamides and reportedly have plasma half-lives of about 4-8 hours. Sulfadiazine and sulfapyridine are generally considered to be intermediate-acting sulfonamides and reportedly have plasma half-lives of about 7-17 hours. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 425
The plasma, urine, and tissue sulfathiazole concentrations were determined at various times following intravenous administration to 12 sheep. The plasma and urine data were consistent with a one-compartment pharmacokinetic model, with an elimination half-life of 1.1 hr...
PMID:903868 Bevill R et al; J Pharm Sci 66 (9): 1297-300 (1977)
Sulfonamides are usually bacteriostatic in action. Sulfonamides interfere with the utilization of p-aminobenzoic acid (PABA) in the biosynthesis of tetrahydrofolic acid (the reduced form of folic acid) cofactors in susceptible bacteria. Sulfonamides are structural analogs of PABA and appear to interfere with PABA utilization by competitively inhibiting the enzyme dihydropteroate synthase, which catalyzes the formation of dihydropteroic acid (a precursor of tetrahydrofolic acid) from PABA and pteridine; however, other mechanism(s) affecting the biosynthetic pathway also may be involved. Compounds such as pyrimethamine and trimethoprim, which block later stages in the synthesis of folic acid, act synergistically with sulfonamides. Only microorganisms that synthesize their own folic acid are inhibited by sulfonamides; animal cells and bacteria which are capable of utilizing folic acid precursors or preformed folic acid are not affected by these drugs. The antibacterial activity of the sulfonamides is reportedly decreased in the presence of blood or purulent body exudates. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 424
The sulfonamides are structural analogs of para-aminobenzoic acid (PABA) and competitively inhibit an enzymatic step (dihydropterate synthetase) during which PABA is incorporated into the synthesis of dihydrofolic acid (folic acid). Because dihydrofolate synthesis is reduced, the levels of tetrahydrofolate (folinic acid) formed from dihydrofolate diminish. Tetrahydrofolate is an essential component of the coenzymes responsible for single carbon metabolism in cells. Acting as antimetabolites to PABA, sulfonamides eventually block, in a complex fashion, several enzymes. These enzymes include those needed for the biogenesis of purine bases; for the transfer of desoxyuridine to thymidine; and for the biosynthesis of methionine, glycine, and formylmethionyl-transfer-RNA. This results in suppression of protein synthesis, impairment of metabolic processes, and inhibition of growth and multiplication of those organisms that cannot use preformed folate. The effect is bacteriostatic, although a bactericidal action is evident at the high concentrations that may be found in urine.
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2076

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

ABOUT THIS PAGE
90
PharmaCompass offers a list of Cerazole API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Cerazole manufacturer or Cerazole supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Cerazole manufacturer or Cerazole supplier.
PharmaCompass also assists you with knowing the Cerazole API Price utilized in the formulation of products. Cerazole API Price is not always fixed or binding as the Cerazole Price is obtained through a variety of data sources. The Cerazole Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Cerazole manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Cerazole, including repackagers and relabelers. The FDA regulates Cerazole manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Cerazole API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Cerazole supplier is an individual or a company that provides Cerazole active pharmaceutical ingredient (API) or Cerazole finished formulations upon request. The Cerazole suppliers may include Cerazole API manufacturers, exporters, distributors and traders.
click here to find a list of Cerazole suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Cerazole DMF (Drug Master File) is a document detailing the whole manufacturing process of Cerazole active pharmaceutical ingredient (API) in detail. Different forms of Cerazole DMFs exist exist since differing nations have different regulations, such as Cerazole USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Cerazole DMF submitted to regulatory agencies in the US is known as a USDMF. Cerazole USDMF includes data on Cerazole's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Cerazole USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Cerazole suppliers with USDMF on PharmaCompass.
Cerazole Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Cerazole GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Cerazole GMP manufacturer or Cerazole GMP API supplier for your needs.
A Cerazole CoA (Certificate of Analysis) is a formal document that attests to Cerazole's compliance with Cerazole specifications and serves as a tool for batch-level quality control.
Cerazole CoA mostly includes findings from lab analyses of a specific batch. For each Cerazole CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Cerazole may be tested according to a variety of international standards, such as European Pharmacopoeia (Cerazole EP), Cerazole JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Cerazole USP).

