



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
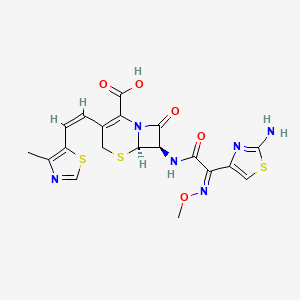

1. Cefditoren, Sodium Salt, (6r-(3(z),6alpha,7beta(z)))-isomer
2. Me 1206
3. Me-1206
1. 104145-95-1
2. Cefditoren [inn]
3. 81qs09v3yw
4. Chebi:59343
5. Cefditoren (inn)
6. (6r,7r)-7-[[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-3-[(z)-2-(4-methyl-1,3-thiazol-5-yl)ethenyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
7. Cefditoren [usan:inn]
8. Unii-81qs09v3yw
9. Cdtr
10. Cefditoren [mi]
11. Cefditoren [vandf]
12. Cefditoren [who-dd]
13. Chembl1743
14. Schembl37473
15. Dtxsid501328012
16. Db01066
17. (6r-(3(z),6alpha,7beta(z)))-7-((2-amino-4-thiazolyl)(methoxyimino)acetyl)amino-3-(2-(4-methyl-5-thiazolyl)ethenyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid
18. C-2467
19. C21546
20. D07639
21. Cefditorene, Antibiotic For Culture Media Use Only
22. (+)-(6r,7r)-7-(2-(2-amino-4-thiazolyl)glyoxylamido)-3-((z)-2-(4-methyl-5-thiazolyl)vinyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7(sup 2)-(z)-(o-methyloxime)
23. (6r,7r)-7-[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetamido]-3-[(z)-2-(4-methyl-1,3-thiazol-5-yl)ethenyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
24. (6r,7r)-7-{[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-3-[(z)-2-(4-methyl-1,3-thiazol-5-yl)ethenyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
25. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-((2-amino-4-thiazolyl)(methoxyimino)acetyl)amino-3-(2-(4-methyl-5-thiazolyl)ethenyl)-8-oxo-, (6r-(3(z),6alpha,7beta(z)))-
26. 7beta-(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetamido-3-[(z)-2-(4-methyl-1,3-thiazol-5-yl)ethenyl]-3,4-didehydrocepham-4-carboxylic Acid
| Molecular Weight | 506.6 g/mol |
|---|---|
| Molecular Formula | C19H18N6O5S3 |
| XLogP3 | 0.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 7 |
| Exact Mass | 506.05008122 g/mol |
| Monoisotopic Mass | 506.05008122 g/mol |
| Topological Polar Surface Area | 242 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 928 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of mild to moderate infections in adults and adolescents (12 years of age or older) which are caused by susceptible strains of microorganisms in acute bacterial exacerbation of chronic bronchitis, community-acquired pneumonia, pharyngitis/tonsillitis, and uncomplicated skin and skin-structure infections.
FDA Label
Cefditoren pivoxil is a prodrug which is hydrolyzed by esterases during absorption, and the drug is distributed in the circulating blood as active cefditoren. Cefditoren is a cephalosporin with antibacterial activity against gram-positive and gram-negative pathogens. Cefditoren is effective against Staphylococcus aureus (methicillin-susceptible strains, including b-lactamase-producing strains), penicillin-susceptible strains of Staphylococcus aureus and Streptococcus pneumoniae, Streptococcus pyogenes, Haemophilus influenzae (including b-lactamase-producing strains), Haemophilus parainfluenzae (including b-lactamase-producing strains), Moraxella catarrhalis (including b-lactamase-producing strains), Streptococcus agalactiae, Streptococcus Groups C and G, and Streptococcus, viridans group (penicillin-susceptible and -intermediate strains).
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DD - Third-generation cephalosporins
J01DD16 - Cefditoren
Absorption
Following oral administration, cefditoren pivoxil is absorbed from the gastrointestinal tract and hydrolyzed to cefditoren by esterases. Under fasting conditions, the estimated absolute bioavailability of cefditoren pivoxil is approximately 14%. The absolute bioavailability of cefditoren pivoxil administered with a low fat meal (693 cal, 14 g fat, 122 g carb, 23 g protein) is 16.1 ± 3.0%.
Route of Elimination
Pivalate is mainly eliminated (>99%) through renal excretion, nearly exclusively as pivaloylcarnitine.
Volume of Distribution
9.3 1.6 L
Clearance
renal cl=4-5 L/h [oral administration]
Hydrolysis of cefditoren pivoxil to its active component, cefditoren, results in the formation of pivalate. Cefditoren is not appreciably metabolized.
Mean terminal elimination half-life is 1.6 ± 0.4 hours in young healthy adults.
The bactericidal activity of cefditoren results from the inhibition of cell wall synthesis via affinity for penicillin-binding proteins (PBPs). Cefditoren is stable in the presence of a variety of b-lactamases, including penicillinases and some cephalosporinases.


