



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe

Canada
0

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
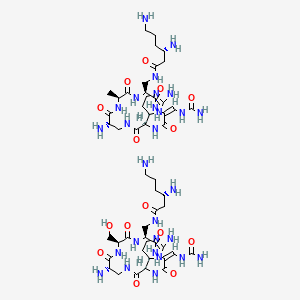

1. Schembl3666
2. Beta-sitosterolacetate
3. Q415909
| Molecular Weight | 1321.4 g/mol |
|---|---|
| Molecular Formula | C50H88N28O15 |
| Hydrogen Bond Donor Count | 27 |
| Hydrogen Bond Acceptor Count | 23 |
| Rotatable Bond Count | 19 |
| Exact Mass | 1320.69839421 g/mol |
| Monoisotopic Mass | 1320.69839421 g/mol |
| Topological Polar Surface Area | 737 Ų |
| Heavy Atom Count | 93 |
| Formal Charge | 0 |
| Complexity | 2470 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 12 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antibiotics, Antitubercular; Antibiotics, Peptide
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Capreomycin is indicated in combination with other antituberculosis medications in the treatment of pulmonary tuberculosis caused by Mycobacterium tuberculosis after failure with the primary medications (streptomycin, isoniazid, rifampin, pyrazinamide, and ethambutol) or when these cannot be used because of toxicity or development of resistant tubercle bacilli. /Included in US product label/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 726
Since bacterial resistance may develop rapidly when capreomycin is administered alone, it should only be administered concurrently with other antituberculosis medications in the treatment of tuberculosis. /NOT included in US product label/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 726
Nephrotoxicity and ototoxicity are the most serious adverse effects of capreomycin. These effects are most likely to occur in patients with renal impairment, in geriatric patients, and in patients who are receiving other nephrotoxic and/or ototoxic drugs.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 565
Renal toxicity may be manifested by tubular necrosis, increases in BUN and nonprotein nitrogen, decreased creatinine clearance, proteinuria, and the presence of casts, erythrocytes, and leukocytes in the urine. The manufacturer states that BUN concentrations increased to greater than 20 mg/dL in 36% of 722 patients receiving capreomycin; BUN concentrations were greater than 30 mg/dL in 10% of patients. There also was depression of PSP excretion and abnormal urine sediment in many patients. Renal toxicity is usually reversible following discontinuation of the drug however, fatal toxic nephritis has occurred rarely. Fatal toxic nephritis was reported in one patient with tuberculosis and portal cirrhosis who had received one month of therapy with capreomycin (1 g daily) in conjunction with aminosalicylic acid; the patient developed renal insufficiency and oliguria and autopsy indicated subsiding acute tubular necrosis. Nephrotoxicity is most closely related to the area under the serum concentration-time curve. Geriatric patients, patients with abnormal renal function or dehydration, and patients receiving other nephrotoxic drugs are at increased risk of developing acute tubular necrosis during capreomycin therapy.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 565
Electrolyte disturbances including alkalosis and decreased serum concentrations of potassium, magnesium, and calcium have also occurred because of renal tubular dysfunction in patients receiving capreomycin. Electrolyte disturbances resembling Bartter's syndrome have been reported in at least one patient.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 565
Capreomycin may produce damage to both the auditory and vestibular portions of the eighth cranial nerve. Damage to auditory function may result in a hearing loss. Rarely, permanent deafness has occurred. Headache, tinnitus, and vertigo have occurred rarely from injury to the vestibular branch of the eighth cranial nerve. The manufacturer states that subclinical auditory loss (5- to 10-decibel loss in the 4000-8000 CPS range) was noted in approximately 11% of 722 patients receiving capreomycin; clinically apparent hearing loss occurred in 3% of patients. Some audiometric changes were reversible; hearing loss that was permanent was not progressive following discontinuance of capreomycin. Damage to the auditory and vestibular divisions of the eighth cranial nerve have generally been associated with capreomycin therapy in patients with impaired renal function or dehydration or those receiving other drugs with additive auditory toxicities; these patients often experience dizziness, tinnitus, vertigo, and a loss of high-tone acuity.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 565
For more Drug Warnings (Complete) data for CAPREOMYCIN (13 total), please visit the HSDB record page.
J - Antiinfectives for systemic use
J04 - Antimycobacterials
J04A - Drugs for treatment of tuberculosis
J04AB - Antibiotics
J04AB30 - Capreomycin
Capreomycin sulfate is not appreciably absorbed from the GI tract and therefore must be given parenterally. Following im administration of a single 1 g dose of capreomycin in healthy adults, peak plasma capreomycin concentrations ranging from 20-47 ug/mL are attained within 1-2 hours (averaging 28 and 32 ug/mL at 1 and 2 hours, respectively); plasma concentrations of the drug average 10 ug/mL at 6 hours and less than 1 ug/mL at 24 hours. Following administration of a single 1-g dose im or by iv infusion over 1 hour, the area under the serum concentration-time curve (AUC) was similar for both routes of administration. However, peak serum capreomycin concentrations after iv infusion were 30% higher than those following IM injection.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 566
/Investigators/ examined the pharmacokinetics of single dose capreomycin (1.0 g) administered intramuscularly and by intravenous infusion (1 hour) in 6 healthy volunteers. The area under the serum concentration versus time curve was similar for the two routes of administration. Capreomycin peak concentrations after intravenous infusion were 30 +/- 47% higher than after intramuscular administration.
US Natl Inst Health; DailyMed. Current Medication Information. Capreomycin (01/2008). Available from, as of July 8, 2008: https://dailymed.nlm.nih.gov/dailymed/about.cfm
Capreomycin does not distribute into CSF. Information is not available on the distribution of capreomycin into other body tissue or fluids. It is not known if the drug crosses the placenta or is distributed into milk.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 566
Capreomycin is excreted mainly unchanged in urine by glomerular filtration. Results of animal studies suggest that small amounts of the drug may also be excreted in bile. Following a single 1g IM dose of capreomycin in adults with normal renal function, approximately 52% of the dose is excreted in urine within 12 hours.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 566
For more Absorption, Distribution and Excretion (Complete) data for CAPREOMYCIN (6 total), please visit the HSDB record page.
Paper chromatographic studies indicated that capreomycin is excreted essentially unaltered. Urine concentrations averaged 1.68 mg/mL (average urine volume, 228 mL) during the 6 hours following a 1-g dose.
US Natl Inst Health; DailyMed. Current Medication Information. Capreomycin (01/2008). Available from, as of July 8, 2008: https://dailymed.nlm.nih.gov/dailymed/about.cfm
The plasma half-life of capreomycin in patients with normal renal function is 4-6 hours. Plasma concentrations of capreomycin are higher and the half-life is prolonged in patients with impaired renal function.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 566
Aminoglycosides are usually bactericidal in action. Although the exact mechanism of action has not been fully elucidated, the drugs appear to inhibit protein synthesis in susceptible bacteria by irreversibly binding to 30S ribosomal subunits. /Aminoglycosides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 67
Capreomycin is an important drug used for TB with multi-drug resistance. A recent study also indicates that this drug possesses unique bactericidal activity against non-replicating TB bacilli among known anti-TB drugs. Thus, there is an urgent need for investigating the full-spectrum action of capreomycin. Here /investigators/ conduct the first microarray-based study on capreomycin using the high-resolution Affymetrix oligonucleotide GeneChip system. The results indicate that capreomycin primarily acts on the information pathways but it also significantly affects cell wall, cell processes, intermediate metabolism and respiration in Mycobacterium tuberculosis. This study not only transcriptionally validates the specific molecular target, 16S rRNA, but also discovers potential new targets of capreomycin, including genes operating at the DNA level, such as Rv0054 (ssb) and Rv3715c (recR), as well as genes involved in cell division like Rv3260c (whiB2). In addition, the nuo gene cluster and the ATP synthase gene cluster are repressed.
PMID:16822547 Fu L, Shinnick T; J Infect 54 (3): 277-84 (2007)


