


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
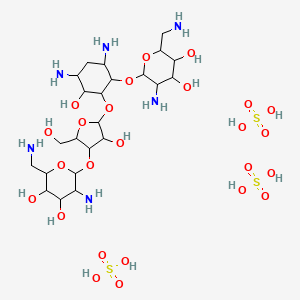

1. Fradiomycin Sulfate
2. Neomycin
3. Neomycin Palmitate
4. Neomycin Sulfate
1. Framycetin (sulfate)
2. Fradiomycin B (sulfate); Neomycin B (sulfate)
3. Neomycin Sulfate
4. 4146-30-9
5. Bcp28374
6. Neomycin Sulfate; Neomycin Sulphate B
| Molecular Weight | 908.9 g/mol |
|---|---|
| Molecular Formula | C23H52N6O25S3 |
| Hydrogen Bond Donor Count | 19 |
| Hydrogen Bond Acceptor Count | 31 |
| Rotatable Bond Count | 9 |
| Exact Mass | 908.21442469 g/mol |
| Monoisotopic Mass | 908.21442469 g/mol |
| Topological Polar Surface Area | 602 Ų |
| Heavy Atom Count | 57 |
| Formal Charge | 0 |
| Complexity | 953 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 19 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 4 |
| 1 of 20 | |
|---|---|
| Drug Name | NEO-SYNALAR |
| Active Ingredient | FLUOCINOLONE ACETONIDE; NEOMYCIN SULFATE |
| Company | MEDIMETRIKS PHARMS (Application Number: A060700) |
| 2 of 20 | |
|---|---|
| Drug Name | NEOMYCIN AND POLYMYXIN B SULFATE |
| Active Ingredient | NEOMYCIN SULFATE; POLYMYXIN B SULFATE |
| Company | WATSON LABS (Application Number: A062664); X GEN PHARMS (Application Number: A065106); X GEN PHARMS (Application Number: A065108) |
| 3 of 20 | |
|---|---|
| Drug Name | NEOSPORIN G.U. IRRIGANT |
| Active Ingredient | NEOMYCIN SULFATE; POLYMYXIN B SULFATE |
| Company | MONARCH PHARMS (Application Number: A060707) |
| 4 of 20 | |
|---|---|
| Drug Name | NEOMYCIN AND POLYMYXIN B SULFATES AND BACITRACIN ZINC |
| Active Ingredient | BACITRACIN ZINC; NEOMYCIN SULFATE; POLYMYXIN B SULFATE |
| Company | AKORN (Application Number: A065088); BAUSCH AND LOMB (Application Number: A064064); PERRIGO CO TENNESSEE (Application Number: A060764) |
| 5 of 20 | |
|---|---|
| Drug Name | NEOSPORIN |
| Active Ingredient | BACITRACIN ZINC; NEOMYCIN SULFATE; POLYMYXIN B SULFATE |
| Company | CASPER PHARMA LLC (Application Number: N050417) |
| 6 of 20 | |
|---|---|
| Drug Name | DEXASPORIN |
| Active Ingredient | DEXAMETHASONE; NEOMYCIN SULFATE; POLYMYXIN B SULFATE |
| Company | BAUSCH AND LOMB (Application Number: A064135) |
| 7 of 20 | |
|---|---|
| Drug Name | MAXITROL |
| Active Ingredient | DEXAMETHASONE; NEOMYCIN SULFATE; POLYMYXIN B SULFATE |
| Company | NOVARTIS PHARMS CORP (Application Number: N050023); NOVARTIS PHARMS CORP (Application Number: N050065); SANDOZ INC (Application Number: A062341) |
| 8 of 20 | |
|---|---|
| Drug Name | NEOMYCIN AND POLYMYXIN B SULFATES AND DEXAMETHASONE |
| Active Ingredient | DEXAMETHASONE; NEOMYCIN SULFATE; POLYMYXIN B SULFATE |
| Company | BAUSCH AND LOMB (Application Number: A064063); PERRIGO CO TENNESSEE (Application Number: A062938) |
| 9 of 20 | |
|---|---|
| Drug Name | NEOMYCIN AND POLYMYXIN B SULFATES AND GRAMICIDIN |
| Active Ingredient | GRAMICIDIN; NEOMYCIN SULFATE; POLYMYXIN B SULFATE |
| Company | AMRING PHARMS (Application Number: A065187); BAUSCH AND LOMB (Application Number: A064047) |
| 10 of 20 | |
|---|---|
| Drug Name | NEOSPORIN |
| Active Ingredient | GRAMICIDIN; NEOMYCIN SULFATE; POLYMYXIN B SULFATE |
| Company | MONARCH PHARMS (Application Number: A060582) |
| 11 of 20 | |
|---|---|
| Drug Name | CORTISPORIN |
| Active Ingredient | HYDROCORTISONE ACETATE; NEOMYCIN SULFATE; POLYMYXIN B SULFATE |
| Company | MONARCH PHARMS (Application Number: N050218) |
| 12 of 20 | |
|---|---|
| Drug Name | CASPORYN HC |
| Active Ingredient | HYDROCORTISONE; NEOMYCIN SULFATE; POLYMYXIN B SULFATE |
| Company | CASPER PHARMA LLC (Application Number: N060613) |
| 13 of 20 | |
|---|---|
| Drug Name | CORTISPORIN |
| Active Ingredient | HYDROCORTISONE; NEOMYCIN SULFATE; POLYMYXIN B SULFATE |
| Company | MONARCH PHARMS (Application Number: N050479) |
| 14 of 20 | |
|---|---|
| Drug Name | NEOMYCIN AND POLYMYXIN B SULFATES AND HYDROCORTISONE |
| Active Ingredient | HYDROCORTISONE; NEOMYCIN SULFATE; POLYMYXIN B SULFATE |
| Company | AMRING PHARMS (Application Number: A065216); AMRING PHARMS (Application Number: A065219); BAUSCH AND LOMB (Application Number: A064053); SANDOZ INC (Application Number: A062423); SANDOZ INC (Application Number: A062488); SANDOZ INC (Application Number: A062874) |
| 15 of 20 | |
|---|---|
| Drug Name | OTICAIR |
| Active Ingredient | HYDROCORTISONE; NEOMYCIN SULFATE; POLYMYXIN B SULFATE |
| Company | BAUSCH AND LOMB (Application Number: A064065) |
| 16 of 20 | |
|---|---|
| Drug Name | BACITRACIN-NEOMYCIN-POLYMYXIN W/ HYDROCORTISONE ACETATE |
| Active Ingredient | BACITRACIN ZINC; HYDROCORTISONE ACETATE; NEOMYCIN SULFATE; POLYMYXIN B SULFATE |
| Company | PERRIGO CO TENNESSEE (Application Number: A062166) |
| 17 of 20 | |
|---|---|
| Drug Name | CORTISPORIN |
| Active Ingredient | BACITRACIN ZINC; HYDROCORTISONE; NEOMYCIN SULFATE; POLYMYXIN B SULFATE |
| Company | MONARCH PHARMS (Application Number: N050168) |
| 18 of 20 | |
|---|---|
| Drug Name | NEOMYCIN AND POLYMYXIN B SULFATES, BACITRACIN ZINC AND HYDROCORTISONE |
| Active Ingredient | BACITRACIN ZINC; HYDROCORTISONE; NEOMYCIN SULFATE; POLYMYXIN B SULFATE |
| Company | AKORN (Application Number: A065213); BAUSCH AND LOMB (Application Number: A064068) |
| 19 of 20 | |
|---|---|
| Drug Name | COLY-MYCIN S |
| Active Ingredient | COLISTIN SULFATE; HYDROCORTISONE ACETATE; NEOMYCIN SULFATE; THONZONIUM BROMIDE |
| Company | ENDO PHARMS INC (Application Number: N050356) |
| 20 of 20 | |
|---|---|
| Drug Name | NEOMYCIN SULFATE |
| Active Ingredient | NEOMYCIN SULFATE |
| Company | BRECKENRIDGE PHARM (Application Number: A065468); LANNETT CO INC (Application Number: A204435); TEVA (Application Number: A060304); X GEN PHARMS (Application Number: A065220) |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Protein Synthesis Inhibitors
Compounds which inhibit the synthesis of proteins. They are usually ANTI-BACTERIAL AGENTS or toxins. Mechanism of the action of inhibition includes the interruption of peptide-chain elongation, the blocking the A site of ribosomes, the misreading of the genetic code or the prevention of the attachment of oligosaccharide side chains to glycoproteins. (See all compounds classified as Protein Synthesis Inhibitors.)


