
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
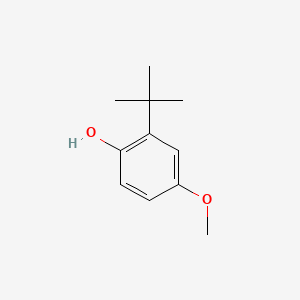

1. Bha
2. Butyl Methoxyphenol
3. Butylhydroxyanisole
4. (1,1-dimethylethyl)-4-methoxyphenol
5. Tenox Bha
6. Nipantiox 1-f
7. Embanox
1. 25013-16-5
2. Phenol, (1,1-dimethylethyl)-4-methoxy-
3. Tert-butyl-4-hydroxyanisole
4. 2(3)-tert-butyl-4-hydroxyanisole
5. Antrancine 12
6. Nipantiox 1-f
7. Tenox Bha
8. Butylohydroksyanizol
9. Butyl Hydroxyanisole
10. Boa (antioxidant)
11. Sustane 1-f
12. Eec No. E320
13. Premerge Plus
14. Phenol, Tert-butyl-4-methoxy-
15. Nipantiox 1f
16. Sustane 1f
17. Nipantiox 1 F
18. Butyl Methoxyphenol
19. Methoxyphenol, Butyl
20. Tert-butylhydroxyanisole
21. Refchem:571819
22. Amif72
23. Amif 72
24. Rek4960k2u
25. Dtxsid7020215
26. Chebi:76359
27. Dtxcid701285159
28. 246-563-8
29. (1,1-dimethylethyl)-4-methoxyphenol
30. 3-tertiary-butyl-4-hydroxyanisole
31. Antioxyne B
32. Butylhydroxyanisole
33. Butylhydroxyanisolum
34. E-320
35. E320
36. Embanox
37. Fema No. 2183
38. Ins No.320
39. Ins-320
40. Protex
41. Tert-butyl-4-methoxyphenol
42. Tert-butyl-p-hydroxyanisole
43. 2-tert-butyl-4-methoxyphenol
44. 3-tert-butyl-4-hydroxyanisole
45. 121-00-6
46. 4-hydroxy-3-tert-butylanisole
47. 2-(tert-butyl)-4-methoxyphenol
48. 3-bha
49. 3-t-butyl-4-hydroxyanisole
50. 3-tert-butyl-p-hydroxyanisole
51. Phenol, 2-(1,1-dimethylethyl)-4-methoxy-
52. 4-methoxy-2-tert-butylphenol
53. 2-(1,1-dimethylethyl)-4-methoxyphenol
54. Phenol, 2-tert-butyl-4-methoxy-
55. Dtxsid7040788
56. Bha
57. Mfcd00040484
58. 4-methoxy-2-tert-butyl-phenol
59. 2-tert-butyl-4-methoxy-phenol
60. 1341-82-8
61. O-tert-butyl-p-methoxyphenol
62. 3-(1,1-dimethylethyl)-4-hydroxyanisole
63. 4-methoxy-6-tert-butylphenol
64. 62rac24292
65. 921-00-6
66. 2-butyl-4-hydroxyanisole
67. Butylhydroxyanisol
68. Ccris 3746
69. Hsdb 2750
70. Einecs 204-442-7
71. Brn 1867499
72. 2-(1,1-dimethylethyl)-4-methoxy-phenol
73. Unii-62rac24292
74. 2-t-butyl-4-methoxyphenol (bha)
75. 3-tert-butyl-4-hydroxyanisole; 2-tert-butyl-4-methoxyphenol; Bha; Butylated Hydroxyanisole Related
76. 2-tert-bha
77. Dsstox_cid_215
78. 2-t-butyl-4-methoxyphenol
79. Butylated Hydroxyanisole/bha
80. Dsstox_rid_75439
81. P-methoxy-o-tert-butylphenol
82. Dsstox_gsid_20215
83. Schembl35535
84. 3-tert-butyl-4-hydroxyanisol
85. Mls000069623
86. Bidd:er0078
87. Dtxcid90215
88. Orb1301848
89. Orb1310293
90. Schembl1695152
91. Schembl6912657
92. Schembl29362042
93. Chebi:76358
94. Msk2301
95. Glxc-07224
96. Hms1610g14
97. Hms2233f16
98. Hms3370c01
99. Albb-023610
100. Msk15041
101. Tox21_201822
102. Tox21_300082
103. Bbl027948
104. Sbb043781
105. Stl377914
106. 2(3)-t-butyl-4-hydroxyanisole;bha
107. 2-tert-butyl-4-methoxyphenol (bha)
108. Akos000120777
109. Ccg-107062
110. Fb32550
111. Hy-w010037
112. Ncgc00159405-02
113. Ncgc00159405-03
114. Ncgc00159405-04
115. Ncgc00159405-05
116. Ncgc00254068-01
117. Ncgc00259371-01
118. As-63590
119. Cas-121-00-6
120. Da-61954
121. Msk2301-1000
122. Smr000058171
123. Sy113469
124. 3-t-butyl-4-hydroxyanisole [hsdb]
125. Cas-25013-16-5
126. Phenol,2-(1,1-dimethylethyl)-4-methoxy-
127. B0723
128. Eu-0072133
129. Ns00009076
130. St45022092
131. En300-20834
132. 3-tert-butyl-4-hydroxyanisole [usp-rs]
133. Ab00514297_09
134. Benzene,1-tert.butyl,2-hydroxy,5-methoxy
135. F116500
136. Brd-k93035859-001-07-6
137. Q27145913
138. Z104483428
139. 3-tert-butyl-4-hydroxyanisole, Tested According To Ph.eur.
140. 3-tert-butyl-4-hydroxyanisole, >=98% (sum Of Isomers, Gc), <=10% 2-bha Basis (gc)
141. 3-tert-butyl-4-hydroxyanisole, United States Pharmacopeia (usp) Reference Standard
142. Inchi=1/c11h16o2/c1-11(2,3)9-7-8(13-4)5-6-10(9)12/h5-7,12h,1-4h
143. Tert-butyl-4-hydroxyanisole (mixture Of 2- And 3-isomer) Solution In Methanol, 1000?g/ml
144. 3-tert-butyl-4-hydroxyanisole, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 180.24 g/mol |
|---|---|
| Molecular Formula | C11H16O2 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | Da |
| Monoisotopic Mass | Da |
| Topological Polar Surface Area | 29.5 |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 160 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues.
Carcinogens
Substances that increase the risk of NEOPLASMS in humans or animals. Both genotoxic chemicals, which affect DNA directly, and nongenotoxic chemicals, which induce neoplasms by other mechanism, are included.
CONCENTRATIONS OF 2-TERT-BUTYL-4-METHOXYPHENOL & 2,2'-DIHYDROXY-3,3'-DI-TERT-BUTYL-5,5'DIMETHOXY-DIPHENYL (DI-BHA) APPEARED AT DIFFERENT TIMES (0.15-24 HR) IN RAT PLASMA & INTESTINE FOLLOWING 2 G/KG SINGLE ORAL ADMIN OF 2-TERT-BUTYL-4-METHOXYPHENOL. PEAK CONCN IN ALL TISSUES ANALYZED WERE OBSERVED WITHIN 1 HR OF ADMIN. IN INTESTINE 2-TERT-BUTYL-4-METHOXYPHENOL LEVELS WERE APPROX 10 TIMES HIGHER THAN DI-BHA; IN PLASMA THEY WERE BETWEEN 100 & 15 TIMES HIGHER. THE RAT INTESTINE IS CAPABLE OF TRANSFORMING IN VIVO 2-TERT-BUTYL-4-METHOXYPHENOL INTO DI-BHA & MAY BE MAJOR SITE WHERE THIS TRANSFORMATION OCCURS.
PMID:6140143 GUARNA A ET AL; DRUG METAB DISPOS 11 (6): 581-4 (1983)
Absorption of BHA from the digestive tract is by passive diffusion.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V5 926
BHA was fed to groups of three beagle dogs at dose levels of 0, 0.3, 3, 30 and 100 mg/kg bw for 1 yr. All animals survived; no pathological lesion was seen, and there was not demonstrable storage of BHA ... .
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V40 137 (1986)
In male and female Sprague-Dawley rats, BHA was absorbed rapidly after oral administration and metabolized.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V40 138 (1986)
When male volunteers were given an oral dose of 50 mg BHA, 27-77% was excreted in the urine as the glucuronide and urinary metabolites. ... Urinary excretion of BHA was maximal within 17 hr and complete by 48 hr. ... When human volunteers were given a single oral dose of 14(C)-labelled BHA (approx 0.5 mg/kg bw), 60-70% of the radioactivity was excreted in the urine within 2 days and 80-86.5% by day 11. ... Four male volunteers were given 30 mg BHA orally and 10 days later
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V40 146 (1986)
FOLLOWING ORAL ADMIN OF 2 G/KG OF 2-TERT-BUTYL-4-METHOXYPHENOL TO RATS, THE METABOLITE 2,2'-DIHYDROXY-3,3'-DI-TERT-BUTYL-5,5'-DIMETHOXYDIPHENYL WAS DETECTED IN PLASMA & TISSUE 0.15-24 HR AFTER ADMIN.
PMID:6140143 GUARNA A ET AL; DRUG METAB DISPOS 11 (6): 581-4 (1983)
DI-BHA (2,2'-DIHYDROXY-3,3'-DI-TERT-BUTYL-5,5'-DIMETHOXYDIPHENYL) WAS ISOLATED AS REACTION PRODUCT OF EITHER COMMERCIAL HORSERADISH PEROXIDASE OR PARTIALLY PURIFIED RAT INTESTINE PEROXIDASE & HYDROGEN PEROXIDE WITH 2-TERT-BUTYL-4-METHOXYPHENOL. CYCLIC COMPOUNDS (SUCH AS DI-BHA) POSSESSING A HYDROXY GROUP IN THE RING ARE COMPETITIVE INHIBITORS WITH RESPECT TO GUAIACOL & NON-COMPETITIVE INHIBITORS WITH RESPECT TO HYDROGEN PEROXIDASE IN A SYSTEM CONTAINING GUAIACOL, HYDROGEN PEROXIDASE & PEROXIDASE.
SGARAGLI G ET AL; BIOCHEM PHARMACOL 29 (5): 763-70 (1980)
In rat, oral doses (0.4 g/kg) are excreted largely in urine, as glucuronide conjugate (72% of dose) with smaller ammounts of ethereal sulfate (14%) and of unchanged BHA (5%). Similar pattern of metabolism is ... seen in rabbit and human ...
Parke, D. V. The Biochemistry of Foreign Compounds. Oxford: Pergamon Press, 1968., p. 166
... Dogs excrete only small amounts of BHA glucuronide in urine (5.5%) and most of dose is excreted as unchanged BHA in feces. Dogs also excrete greater proportion as ethereal sulfate (23% of dose), and ... form hydroxylated and demethylated metabolites ... not detected in human urine.
Parke, D. V. The Biochemistry of Foreign Compounds. Oxford: Pergamon Press, 1968., p. 167
In male and female Sprague-Dawley rats, BHA was absorbed rapidly after oral administration and metabolized. The main metabolites were 4-O-conjugates: the O-sulfate and the O-glucuronide ... (No data on absorption via the skin were available to the Working Group.)
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V40 138 (1986)
After administration of a single oral dose of 1000 mg BHA to New Zealand white rabbits, 46% of the dose was excreted in the urine as glucuronides, 9% as ethereal sulfates and 6% as free phenols. Excretion of glucuronides was inversely dose dependent: 60% was recovered as glucuronides after a dose of 500 mg and 84% after 250 mg. Recovery of BHA as glucuronide after repeated dosing (three or four doses) was lower than that after a single dose ... .
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V40 138 (1986)
ADMIN OF 2(3)-TERT-BUTYL-4-HYDROXYANISOLE (BHA) TO RODENTS PROTECTS A VARIETY OF TARGET TISSUES AGAINST PRODUCTION OF TUMORS BY WIDE RANGE OF CHEMICAL CARCINOGENS. BHA REDUCES LEVELS OF MUTAGENIC METABOLITES PRODUCED FROM BENZO(A)PYRENE & NUMEROUS THERAPEUTIC AGENTS IN VIVO; IT ELEVATES HEPATIC ACTIVITIES OF MICROSOMAL EPOXIDE HYDRATASE & CYTOSOL GLUTATHIONE S-TRANSFERASE; IT ALTERS ACTIVITIES OF OTHER HEPATIC ENZYMES & AFFECTS LEVELS OF SOME HEPATIC CATALYTIC CONSTITUENTS; & IT INCREASES CONCN OF NONPROTEIN THIOL COMPOUNDS IN LIVER & SEVERAL OTHER TISSUES. /BHA/
PMID:455282 BENSON AM ET AL; CANCER RES 39 (8): 2971-7 (1979)
EFFECTS OF BUTYLATED HYDROXYANISOLE (BHA) ON ARYL HYDROCARBON HYDROXYLASE (AHH) ACTIVITES IN LIVER, LUNG & SKIN OF RATS & MICE WERE STUDIED TO EXAMINE POSSIBLE MECHANISMS OF ANTICARCINOGENIC ACTIONS. AHH INDUCERS, 3-METHYLCHOLANTHRENE, PHENOBARBITAL &2,3,7,8-TETRACHLORODIBENZO-P-DIOXIN WERE USED. IT WAS OBSERVED THAT 2-TERT-BUTYL-4-HYDROXYANISOLE, 3-TERT-BUTYL-4-HYDROXYANISOLE & BHA COMMERCIAL MIXTURE (85% 3-BHA & 15% 2-BHA) HAD ABOUT THE SAME POTENCY IN INHIBITING MICROSOMAL AHH, ALTHOUGH THE 2-ISOMER APPEARED TO BE SLIGHTLY MORE INHIBITORY. DATA SUGGEST THAT INHIBITORY ACTION OBSERVED IN COMMERCIAL BHA IS DUE TO THE COMBINED ACTION OF BOTH ISOMERS & IS DEPENDENT ON SPECIES OF ANIMAL, TISSUE TYPES & TREATMENT WITH INDUCERS.
YANG CS ET AL; CHEM BIOL INTERACTIONS 37 (3): 337-50 (1981)
/Both BHA and BHT have chemopreventive action when/ administered together with several carcinogens affecting diverse target organs. The antioxidants increase detoxifying enzymes for carcinogens, and many act as free radical trapping agents. The structure of these agents does not suggest a likely electrophile and tests for genotoxicity have been uniformly negative. Both BHA and BHT have been shown to inhibit cell-to-cell communication in culture. /BHA/
Amdur, M.O., J. Doull, C.D. Klaasen (eds). Casarett and Doull's Toxicology. 4th ed. New York, NY: Pergamon Press, 1991., p. 187
Estrogen metabolism-mediated oxidative stress is suggested to play an important role in estrogen-induced breast carcinogenesis. We have earlier demonstrated that antioxidants, vitamin C (Vit C) and butylated hydroxyanisole (BHA) inhibit 17beta-estradiol (E2)-mediated oxidative stress and oxidative DNA damage, and breast carcinogenesis in female August Copenhagen Irish (ACI) rats. The objective of the present study was to characterize the mechanism by which above antioxidants prevent DNA damage during breast carcinogenesis. Female ACI rats were treated with E2; Vit C; Vit C+E2; BHA; and BHA+E2 for up to 240 days. mRNA and protein levels of a DNA repair enzyme 8-Oxoguanine DNA glycosylase (OGG1) and a transcription factor NRF2 were quantified in the mammary and mammary tumor tissues of rats after treatment with E2 and compared with that of rats treated with antioxidants either alone or in combination with E2. The expression of OGG1 was suppressed in mammary tissues and in mammary tumors of rats treated with E2. Expression of NRF2 was also significantly suppressed in E2-treated mammary tissues and in mammary tumors. Vitamin C or BHA treatment prevented E2-mediated decrease in OGG1 and NRF2 levels in the mammary tissues. Chromatin immunoprecipitation analysis confirmed that antioxidant-mediated induction of OGG1 was through increased direct binding of NRF2 to the promoter region of OGG1. Studies using silencer RNA confirmed the role of OGG1 in inhibition of oxidative DNA damage. Our studies suggest that antioxidants Vit C and BHA provide protection against oxidative DNA damage and E2-induced mammary carcinogenesis, at least in part, through NRF2-mediated induction of OGG1.
PMID:23697596 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3665669 Singh B et al; BMC Cancer 13: 253 (2013)
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE

