



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
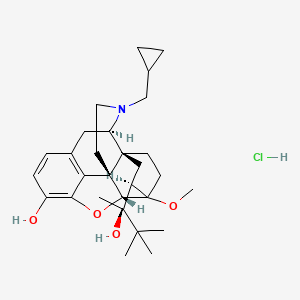

1. 6029 M
2. 6029-m
3. 6029m
4. Buprenex
5. Buprenorphine
6. Buprenorphine Hydrochloride
7. Buprex
8. Hydrochloride, Buprenorphine
9. Prefin
10. Rx 6029 M
11. Rx-6029-m
12. Rx6029m
13. Subutex
14. Temgsic
15. Temgesic
1. Buprenorphine Hydrochloride
2. Ncgc00247733-01
3. Dsstox_cid_28831
4. Dsstox_rid_83100
5. Dsstox_gsid_48905
6. Tox21_112899
7. Cas-53152-21-9
| Molecular Weight | 504.1 g/mol |
|---|---|
| Molecular Formula | C29H42ClNO4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Exact Mass | 503.2802365 g/mol |
| Monoisotopic Mass | 503.2802365 g/mol |
| Topological Polar Surface Area | 62.2 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 869 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Buprenorphine hydrochloride |
| Drug Label | Buprenorphine hydrochloride sublingual tablets contains buprenorphine HCl.Buprenorphine is a partial agonist at the mu-opioid receptor and an antagonist at the kappa-opioid receptor. Buprenorphine is a Schedule III narcotic under the Controlled Subst... |
| Active Ingredient | Buprenorphine hydrochloride |
| Dosage Form | Injectable; Tablet |
| Route | Sublingual; Injection |
| Strength | eq 2mg base; eq 0.3mg base/ml; eq 8mg base |
| Market Status | Prescription |
| Company | Hospira; Ethypharm; Luitpold; Eurohlth Intl; Roxane; Barr |
| 2 of 2 | |
|---|---|
| Drug Name | Buprenorphine hydrochloride |
| Drug Label | Buprenorphine hydrochloride sublingual tablets contains buprenorphine HCl.Buprenorphine is a partial agonist at the mu-opioid receptor and an antagonist at the kappa-opioid receptor. Buprenorphine is a Schedule III narcotic under the Controlled Subst... |
| Active Ingredient | Buprenorphine hydrochloride |
| Dosage Form | Injectable; Tablet |
| Route | Sublingual; Injection |
| Strength | eq 2mg base; eq 0.3mg base/ml; eq 8mg base |
| Market Status | Prescription |
| Company | Hospira; Ethypharm; Luitpold; Eurohlth Intl; Roxane; Barr |
Sixmo is indicated for substitution treatment for opioid dependence in clinically stable adult patients who require no more than 8 mg/day of sublingual buprenorphine, within a framework of medical, social and psychological treatment.
Treatment of opioid dependence
Analgesics, Opioid
Compounds with activity like OPIATE ALKALOIDS, acting at OPIOID RECEPTORS. Properties include induction of ANALGESIA or NARCOSIS. (See all compounds classified as Analgesics, Opioid.)
Narcotic Antagonists
Agents inhibiting the effect of narcotics on the central nervous system. (See all compounds classified as Narcotic Antagonists.)
Narcotics
Agents that induce NARCOSIS. Narcotics include agents that cause somnolence or induced sleep (STUPOR); natural or synthetic derivatives of OPIUM or MORPHINE or any substance that has such effects. They are potent inducers of ANALGESIA and OPIOID-RELATED DISORDERS. (See all compounds classified as Narcotics.)
N07BC01








