


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
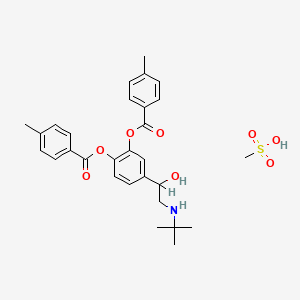

1. Bitolterol
2. Bitolterol Methanesulfonate
3. S-1540
4. Tornalate
5. Win 32784
1. 30392-41-7
2. Tornalate
3. Bitolterol Mesilate
4. Bitolterol Methanesulfonate
5. Bitolterol Mesilat
6. Bitolterol (mesylate)
7. Bitolterol Mesylate [usan]
8. (tert-butyl)[beta-hydroxy-3,4-bis(p-toluoyloxy)phenethyl]ammonium Methanesulphonate
9. Win 32784
10. Win-32784
11. Win 32,784
12. 4-(2-(tert-butylamino)-1-hydroxyethyl)-o-phenylene Di-p-toluate Methanesulfonate (salt)
13. 4e53t3611u
14. Effectin
15. Bitolterol Mesilate (jan)
16. Bitolterol Mesylate (usan)
17. [4-[2-(tert-butylamino)-1-hydroxyethyl]-2-(4-methylbenzoyl)oxyphenyl] 4-methylbenzoate;methanesulfonic Acid
18. Benzoic Acid, 4-methyl-, 4-(2-((1,1-dimethylethyl)amino)-1-hydroxyethyl)-1,2-phenylene Ester Methanesulfonate (salt)
19. Bitolterol Mesilate [jan]
20. Biterol; Bitoterol Methanesulfonate; Effectin; Tornalate; Win 32784
21. Chebi:3134
22. (tert-butyl)(beta-hydroxy-3,4-bis(p-toluoyloxy)phenethyl)ammonium Methanesulphonate
23. Einecs 250-177-5
24. Asmalene
25. Produrol
26. Unii-4e53t3611u
27. Tornalate (tn)
28. 4-(2-(t-butylamino)-1-hydroxyethyl)-o-phenylene Di-p-toluate Mesilate
29. S-1540
30. Schembl4371
31. Alpha((tert-butylamino)methyl)-3,4-dihydroxybenzyl Alcohol 3,4-di-p-toluate Methanesulfonate (salt)
32. Chembl1200405
33. Dtxsid80952735
34. Bitolterol Mesylate [vandf]
35. Bitolterol Mesilate [mart.]
36. Bitolterol Mesilate [who-dd]
37. Bitolterol Methanesulfonate [mi]
38. Bitolterol Mesylate [orange Book]
39. P-toluic Acid, 4-(2-(tert-butylamino)-1-hydroxyethyl)-o-phenylene Ester, Methanesulfonate(salt)
40. D00684
41. Q27105949
42. .alpha.((tert-butylamino)methyl)-3,4-dihydroxybenzyl Alcohol 3,4-di-p-toluate Methanesulfonate (salt)
43. .alpha.((tert-butylamino)methyl)-3,4-dihydroxybenzyl Alcohol 3,4-di-p-toluate Methanesulphonate (salt)
44. 4-(2-(tert-butylamino)-1-hydroxyethyl)-1,2-phenylene Bis(4-methylbenzoate) Methanesulfonate (salt)
45. 4-(2-(tert-butylamino)-1-hydroxyethyl)-1,2-phenylene Bis(4-methylbenzoate) Methanesulphonate (salt)
46. 4-(2-(tert-butylamino)-1-hydroxyethyl)-o-phenylene Di-p-toluate Methanesulphonate (salt)
47. 4-methyl-benzoic Acid 1,1'-[4-[2-[(1,1-dimethylethyl)amino]-1-hydroxyethyl]-1,2-phenylene] Ester Methanesulfonate
48. Alpha ((t-butylamino)methyl)-3,4-dihydroxybenzyl Alcohol 3,4-di(p-toluate) Methanesulfonate
49. Alpha ((t-butylamino)methyl)-3,4-dihydroxybenzyl Alcohol 3,4-di(p-toluate) Methanesulphonate
50. Benzoic Acid, 4-methyl-, 4-(2-((1,1-dimethylethyl)amino)-1-hydroxyethyl)-1,2-phenylene Ester Methanesulphonate (salt)
51. Benzoic Acid, 4-methyl-, 4-(2-((1,1-dimethylethyl)amino)-1-hydroxyethyl)-1,2-phenylene Ester, Methanesulfonate (1:1)
52. Benzoic Acid, 4-methyl-, 4-(2-((1,1-dimethylethyl)amino)-1-hydroxyethyl)-1,2-phenylene Ester, Methanesulphonate (1:1)
| Molecular Weight | 557.7 g/mol |
|---|---|
| Molecular Formula | C29H35NO8S |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 10 |
| Exact Mass | 557.20833825 g/mol |
| Monoisotopic Mass | 557.20833825 g/mol |
| Topological Polar Surface Area | 148 Ų |
| Heavy Atom Count | 39 |
| Formal Charge | 0 |
| Complexity | 751 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Adrenergic beta-2 Receptor Agonists
Compounds bind to and activate ADRENERGIC BETA-2 RECEPTORS. (See all compounds classified as Adrenergic beta-2 Receptor Agonists.)
Bronchodilator Agents
Agents that cause an increase in the expansion of a bronchus or bronchial tubes. (See all compounds classified as Bronchodilator Agents.)


