



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
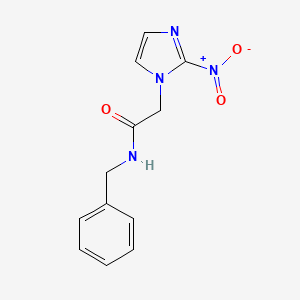

1. 2-nitroimidazole Benznidazole
2. Benzonidazole
3. Radanil
4. Ro 7-1051
1. 22994-85-0
2. Radanil
3. Benznidazol
4. Benzonidazole
5. Benzonidazol
6. Benznidazolum
7. Rochagan
8. Ro 07-1051
9. N-benzyl-2-(2-nitroimidazol-1-yl)acetamide
10. 1h-imidazole-1-acetamide, 2-nitro-n-(phenylmethyl)-
11. N-benzyl-2-(2-nitro-1h-imidazol-1-yl)acetamide
12. N-benzyl-2-nitroimidazole-1-acetamide
13. N-benzyl-2-nitro-1h-imidazole-1-acetamide
14. N-benzyl-2-nitroimidazol-1-yl-acetamide
15. 2-nitro-n-(phenylmethyl)-1h-imidazole-1-acetamide
16. Ro 71051
17. Imidazole-1-acetamide, N-benzyl-2-nitro-
18. Yc42nrj1zd
19. Chembl110
20. Nsc-299972
21. Ro-7-1051
22. Mmv688773
23. Nsc299972
24. Ncgc00166238-01
25. Ro 07-1051;ro 71051
26. Ro-07-1051
27. Dsstox_cid_26570
28. Dsstox_rid_81729
29. Dsstox_gsid_46570
30. Benznidazole [inn]
31. Benznidazol [inn-spanish]
32. Benznidazolum [inn-latin]
33. Smr000857153
34. Nsc 299972
35. Cas-22994-85-0
36. Ccris 2200
37. Sr-01000841264
38. Unii-yc42nrj1zd
39. N-benzyl-2-nitro-1-imidazoleacetamide
40. Brn 0551486
41. Ragonil
42. Acetamide, N-benzyl-2-(nitroimidazol-1-yl)-
43. Benznidazole (tn)
44. Rochagan (tn)
45. Radanil (tn)
46. Benznidazole [mi]
47. Benznidazole (usan/inn)
48. Benznidazole [usan:inn]
49. Benznidazole [usan]
50. Schembl45081
51. Benznidazole [mart.]
52. Mls001332409
53. Mls001332410
54. Mls001360496
55. Benznidazole [who-dd]
56. Benznidazole [who-ip]
57. Dtxsid9046570
58. Schembl22493029
59. Zinc56949
60. Chebi:133833
61. Benznidazole [orange Book]
62. Hms2233g13
63. Hms3369c11
64. Hy-b1548
65. Tox21_112364
66. Bdbm50089916
67. Benznidazolum [who-ip Latin]
68. Mfcd00243089
69. S3741
70. Akos015916722
71. Akos024283499
72. Tox21_112364_1
73. Ccg-267054
74. Db11989
75. Ncgc00166238-02
76. As-68694
77. Cs-0013411
78. Ft-0662547
79. Benznidazol (ro 07-1051; Ro 71051)
80. C74184
81. D02489
82. N-benzyl-2-(2-nitro-imidazol-1-yl)-acetamide
83. N-benzyl-2-(2-nitro-1himidazol-1-yl)acetamide
84. A912716
85. N-benzyl-2-(2-nitro-1h-imidazol-5-yl)acetamide
86. N-benzyl-2-nitro-1h-imidazole-1-acetamide, 97%
87. Q425300
88. 2-(2-nitroimidazol-1-yl)-n-(phenylmethyl)acetamide
89. J-014932
90. Sr-01000841264-3
91. Sr-01000841264-4
92. 1h-imidazole-1-acetamide,2-nitro-n-(phenylmethyl)-
93. Brd-k56156805-001-05-4
94. 1h-imidazole-1-acetamide, 2-nitro-n-(phenylmethyl)- [
95. N-benzyl-2-(2-nitro-imidazol-1-yl)-acetamide (benznidazole)
96. Pyridinium,1-dodecyl-2-[(hydroxyimino)methyl]-,iodide(1:1)
| Molecular Weight | 260.25 g/mol |
|---|---|
| Molecular Formula | C12H12N4O3 |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 260.09094026 g/mol |
| Monoisotopic Mass | 260.09094026 g/mol |
| Topological Polar Surface Area | 92.7 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 325 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For use in the treatment of Chagas disease in children 2-12 years of age.
Benznidazole is a trypanocidal agent which kills the causative organism in Chagas disease, *Trypanosoma cruzi*.
Mutagens
Chemical agents that increase the rate of genetic mutation by interfering with the function of nucleic acids. A clastogen is a specific mutagen that causes breaks in chromosomes. (See all compounds classified as Mutagens.)
Trypanocidal Agents
Agents destructive to the protozoal organisms belonging to the suborder TRYPANOSOMATINA. (See all compounds classified as Trypanocidal Agents.)
Immunosuppressive Agents
Agents that suppress immune function by one of several mechanisms of action. Classical cytotoxic immunosuppressants act by inhibiting DNA synthesis. Others may act through activation of T-CELLS or by inhibiting the activation of HELPER CELLS. While immunosuppression has been brought about in the past primarily to prevent rejection of transplanted organs, new applications involving mediation of the effects of INTERLEUKINS and other CYTOKINES are emerging. (See all compounds classified as Immunosuppressive Agents.)
P - Antiparasitic products, insecticides and repellents
P01 - Antiprotozoals
P01C - Agents against leishmaniasis and trypanosomiasis
P01CA - Nitroimidazole derivatives
P01CA02 - Benznidazole
Absorption
Benznidazole has a bioavailability of 91.7% and a Tmax of 2.93 h.
Route of Elimination
The metabolites of benznidazole appear to be primarily exreted in the urine.
Volume of Distribution
The apparent volume of distribution is 39.19 L.
Clearance
The apparent oral clearance is 2.04 L/h.
Benznidazole is metabolized by nitroreductases in *Trypanosoma cruzi* and by cytochrome P450 enzymes.
The half life of elimination is 13.27 h.
Benznidazole is thought to be reduced to various electrophilic metabolites by nitroreductases present in *Trypanosoma cruzi*. These metabolites likely bind to proteins, lipids, DNA, and RNA resulting in damage to these macromolecules. Benznidazole has been found to increase trypanosomal death through interferon- which is likely present in increased amounts due to inflammation caused by macromolecule damage. DNA in parasites affected by benznidazole has been found to undergo extensive unpacking with overexpression of DNA repair proteins supporting the idea of DNA damage contributing to the mechanism of the drug.






