



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
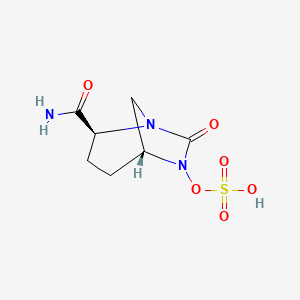

1. Nxl 104
2. Nxl-104
3. Nxl104
1. 1192500-31-4
2. Avibactam Free Acid
3. Nxl104
4. Ave-1330a Free Acid
5. Avibactam (free Acid)
6. Nxl-104 Free Acid
7. Chembl1689063
8. Chebi:85984
9. 06mfo7817i
10. [(2s,5r)-2-carbamoyl-7-oxo-1,6-diazabicyclo[3.2.1]octan-6-yl] Hydrogen Sulfate
11. 396731-14-9
12. (2s,5r)-7-oxo-6-(sulfooxy)-1,6-diazabicyclo[3.2.1]octane-2-carboxamide
13. 1,6-diazabicyclo(3.2.1)octane-2-carboxamide, 7-oxo-6-(sulfooxy)-, (1r,2s,5r)-rel-
14. (1r,2s,5r)-2-carbamoyl-7-oxo-1,6-diazabicyclo[3.2.1]octan-6-yl Hydrogen Sulfate
15. Avibactam [inn]
16. Avibactam [usan:inn]
17. Unii-06mfo7817i
18. Avibactamfreeacid
19. Sulfuric Acid, Mono((1r,2s,5r)-2-(aminocarbonyl)-7-oxo-1,6-diazabicyclo(3.2.1)oct-6-yl) Ester
20. Sulfuric Acid, Mono[(1r,2s,5r)-2-(aminocarbonyl)-7-oxo-1,6-diazabicyclo[3.2.1]oct-6-yl] Ester
21. Unii-7352665165
22. Avibactam [mi]
23. Avibactam [usan]
24. Avibactam [who-dd]
25. Schembl1666807
26. Avibactam, (+/-)-
27. Gtpl10761
28. Dtxsid901026066
29. Amy24028
30. Avi
31. Zavicefta Component Avibactam
32. Zinc9302239
33. Bdbm50339145
34. Avibactam Component Of Zavicefta
35. Cs-0593
36. Db09060
37. Ac-35749
38. Hy-14879
39. E80372
40. Q15410251
41. Trans-7-oxo-6-(sulfooxy)-1,6-diazabicyclo[3.2.1]octan-2-carboxamide
42. (1r,2s,5r)-7-oxo-6-sulfooxy-1,6-diazabicyclo(3.2.1)octane-2-carboxamide
43. 794508-22-8
| Molecular Weight | 265.25 g/mol |
|---|---|
| Molecular Formula | C7H11N3O6S |
| XLogP3 | -1.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 265.03685625 g/mol |
| Monoisotopic Mass | 265.03685625 g/mol |
| Topological Polar Surface Area | 139 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 457 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
AVYCAZ (ceftazidime-avibactam), in combination with metronidazole, is indicated for the treatment of complicated intra-abdominal infections caused by the following susceptible microorganisms: Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Providencia stuartii, Enterobacter cloacae, Klebsiella oxytoca, and Pseudomonas aeruginosa in patients 18 years or older. AVYCAZ is also indicated for the treatment of complicated urinary tract infections including pyelonephritis caused by the following susceptible microorganisms: Escherichia coli, Klebsiella pneumoniae, Citrobacter koseri, Enterobacter aerogenes, Enterobacter cloacae, Citrobacter freundii, Proteus spp., and Pseudomonas aeruginosa in patients 18 years or older.
FDA Label
beta-Lactamase Inhibitors
Endogenous substances and drugs that inhibit or block the activity of BETA-LACTAMASES. (See all compounds classified as beta-Lactamase Inhibitors.)
Route of Elimination
Avibactam and ceftazidime are excreted mainly by the kidneys.
Volume of Distribution
The steady state volumes of distribution of avibactam and ceftazidime is 22.2L and 17L respectively.
Clearance
Avibactam and ceftazidime has a clearance of ~12L/h and ~7L/h respectively.
No metabolism of avibactam was observed in human liver preparations. Unchanged avibactam is the major drug-related component in human plasma and urine. 80-90% of ceftazidime is eliminated as unchanged .
Ceftazidime-avibactam has a half life of ~2.7-3.0 hours.
Avibactam is a non- lactam -lactamase inhibitor that inactivates some -lactamases (Ambler class A -lactamases, including Klebsiella pneumoniae carbapenemases, Ambler class C and some Ambler class D -lactamases) by a unique covalent and reversible mechanism, and protects ceftazidime from degradation by certain -lactamases. Avibactam rapidly reaches the periplasm of bacteria at high enough concentrations to restore activity of ceftazidime against ceftazidime-resistant, -lactamase-producing strains. Avibactam does not decrease the activity of ceftazidime against ceftazidime susceptible organisms.


