



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
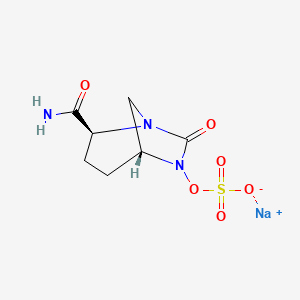

1. Avibactam
2. Nxl 104
3. Nxl-104
4. Nxl104
1. 1192491-61-4
2. Avibactam Sodium Salt
3. Nxl 104
4. Nxl-104
5. Avibactam (sodium)
6. Ave-1330a
7. Avibactam Sodium [usan]
8. Chebi:85982
9. Ave-1330a Sodium
10. C8sm6irw7g
11. 9v824p8tai
12. Sodium (1r,2s,5r)-2-carbamoyl-7-oxo-1,6-diazabicyclo[3.2.1]octan-6-yl Sulfate
13. Sodium (2s,5r)-2-carbamoyl-7-oxo-1,6-diazabicyclo[3.2.1]octan-6-yl Sulfate
14. 396731-20-7
15. Sodium;[(2s,5r)-2-carbamoyl-7-oxo-1,6-diazabicyclo[3.2.1]octan-6-yl] Sulfate
16. 1,6-diazabicyclo(3.2.1)octane-2-carboxamide, 7-oxo-6-(sulfooxy)-, Monosodium Salt, (1r,2s,5r)-rel-
17. Sulfuric Acid Mono[(1r,2s,5r)-2-(aminocarbonyl)-7-oxo-1,6-diazabicyclo[3.2.1]oct-6-yl] Ester Sodium Salt
18. Sulfuric Acid, Mono((1r,2s,5r)-2-(aminocarbonyl)-7-oxo-1,6-diazabicyclo(3.2.1)oct-6-yl) Ester, Sodium Salt (1:1)
19. Sulfuric Acid, Mono((1r,2s,5r)-2-(aminocarbonyl)-7-oxo-1,6-diazabicyclo(3.2.1)oct-6-yl) Ester, Sodium Salt (1:1), Rel-
20. Avibactam Sodium [jan]
21. Unii-9v824p8tai
22. Unii-c8sm6irw7g
23. Avibactam Sodium; Nxl-104
24. Chembl2107817
25. Schembl14247700
26. Avibactam Sodium [who-dd]
27. Bdbm159600
28. Dtxsid701027694
29. Avibactam Sodium Salt [mi]
30. Ave-1330
31. Ex-a2293
32. Avibactam Sodium, (+/-)-
33. Bdbm50512951
34. Hy-14879a
35. Mfcd28900719
36. Akos030243377
37. Avibactam Sodium [orange Book]
38. Ccg-267346
39. Avycaz Component Avibactam Sodium
40. Ac-29295
41. As-74970
42. Avibactam Sodium Component Of Avycaz
43. N-9895
44. Nxl 104, Antibiotic For Culture Media Use Only
45. Us9035062, 23
46. A892614
47. Q27158832
48. Sodium(1r,2s,5r)-2-carbamoyl-7-oxo-1,6-diazabicyclo[3.2.1]octan-6-ylsulfate
49. (2s,5r)-7-oxo-6-(sulfooxy)-1,6-diazabicyclo[3.2.1]octane-2-carboxamide Monosodium Salt;avibactam Sodium
50. 790235-32-4
51. Sodium ({[(2s,5r)-2-carbamoyl-7-oxo-1,6-diazabicyclo[3.2.1]octan-6-yl]oxy}sulfonyl)oxidanide
| Molecular Weight | 287.23 g/mol |
|---|---|
| Molecular Formula | C7H10N3NaO6S |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 287.01880050 g/mol |
| Monoisotopic Mass | 287.01880050 g/mol |
| Topological Polar Surface Area | 141 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 462 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
beta-Lactamase Inhibitors
Endogenous substances and drugs that inhibit or block the activity of BETA-LACTAMASES. (See all compounds classified as beta-Lactamase Inhibitors.)


