



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
0
0
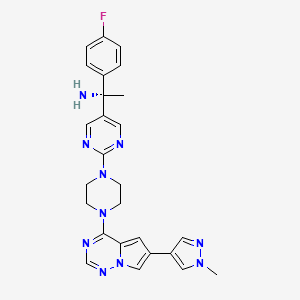

1. (1s)-1-(4-fluorophenyl)-1-(2-(4-(6-(1-methyl-1h-pyrazol-4-yl)pyrrolo(2,1-f)(1,2,4)triazin-4-yl)-1-piperazinyl)-5-pyrimidinyl)ethanamine
2. 5-pyrimidinemethanamine, Alpha-(4-fluorophenyl)-alpha-methyl-2-(4-(6-(1-methyl-1h-pyrazol-4-yl)pyrrolo(2,1-f)(1,2,4)triazin-4-yl)-1-piperazinyl)-, (alphas)-
3. Ayvakit
4. Blu-285
1. Blu-285
2. 1703793-34-3
3. Ayvakit
4. Avapritinib [inn]
5. Blu285
6. (1s)-1-(4-fluorophenyl)-1-[2-[4-[6-(1-methylpyrazol-4-yl)pyrrolo[2,1-f][1,2,4]triazin-4-yl]piperazin-1-yl]pyrimidin-5-yl]ethanamine
7. 513p80b4yj
8. C-366
9. 70c366
10. X720776
11. X-720776
12. Ayvakyt
13. Mfcd31544325
14. Ayvakit (tn)
15. Avapritinib [mi]
16. Avapritinib (usan/inn)
17. Avapritinib [usan]
18. Avapritinib (blu-285)
19. Blu-285 (avapritinib)
20. Avapritinib [who-dd]
21. Unii-513p80b4yj
22. Chembl4204794
23. Schembl16652297
24. Gtpl10368
25. Avapritinib [orange Book]
26. Blu 285
27. Bdbm469269
28. Dtxsid301027935
29. Amy16753
30. Ex-a1366
31. Us10807985, Compound 44
32. Nsc801082
33. S8553
34. Akos037648993
35. Ccg-269677
36. Cs-7577
37. Db15233
38. Nsc-801082
39. (s)-1-(4-fluorophenyl)-1-(2-(4-(6-(1-methyl-1h-pyrazol-4-yl)pyrrolo[2,1-f][1,2,4]triazin-4-yl)piperazin-1-yl)pyrimidin-5-yl)ethan-1-amine
40. Ac-31598
41. Bb166456
42. Bs-16206
43. Hy-101561
44. D11279
45. Q29213676
46. (1s)-1-(4-fluorophenyl)-1-(2-(4-(6-(1-methyl-1h-pyrazol-4-yl)pyrrolo(2,1-f)(1,2,4)triazin-4-yl)-1-piperazinyl)-5-pyrimidinyl)ethanamine
47. (1s)-1-(4-fluorophenyl)-1-(2-(4-(6-(1-methyl-1h-pyrazol-4-yl)pyrrolo(2,1-f)(1,2,4)triazin-4-yl)piperazin-1-yl)pyrimidin-5-yl)ethan-1-amine
48. (s)-1-(4-fluorophenyl)-1-(2-(4-(6-(1-methyl-1h-pyrazol-4-yl)pyrrolo(2,1-f)(1,2,4)triazin-4-yl)piperazin-yl)pyrimidin-5-yl)ethan-1-amine
49. (s)-1-(4-fluorophenyl)-1-(2-(4-(6-(1-methyl-1h-pyrazol-4-yl)pyrrolo[2,1-f][1,2,4]triazin-4-yl)piperazin-1-yl)pyrimidin-5-yl)ethanamine
50. 5-pyrimidinemethanamine, .alpha.-(4-fluorophenyl)-.alpha.-methyl-2-(4-(6-(1-methyl-1h-pyrazol-4-yl)pyrrolo(2,1-f)(1,2,4)triazin-4-yl)-1-piperazinyl)-, (.alpha.s)-
| Molecular Weight | 498.6 g/mol |
|---|---|
| Molecular Formula | C26H27FN10 |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 5 |
| Exact Mass | 498.24041907 g/mol |
| Monoisotopic Mass | 498.24041907 g/mol |
| Topological Polar Surface Area | 106 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 752 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Avapritinib is indicated for the treatment of unresectable, metastatic gastrointestinal stromal tumors with a platelet-derived growth factor receptor alpha exon 18 mutation.
Treatment of mastocytosis
Treatment of all conditions included in the category of malignant neoplasms (except haematopoietic and lymphoid tissue neoplasms)
Ayvakyt is indicated as monotherapy for the treatment of adult patients with unresectable or metastatic gastrointestinal stromal tumours (GIST) harbouring the platelet-derived growth factor receptor alpha (PDGFRA) D842V mutation.
Avapritinib is a selective kinase inhibitor that negatively modulates the action of cell transporters to resensitize them to other chemotherapies. It has a long duration of action as it is given once daily. Patients should be counselled regarding the risk of intracranial hemorrhage, CNS effects, and embryo-fetal toxicity.
L01EX18
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EX - Other protein kinase inhibitors
L01EX18 - Avapritinib
Absorption
A 300mg oral dose of avapritinib reaches a Cmax of 813ng/mL with a Tmax of 2.0-4.1h and an AUC of 15400h\*ng/mL.
Route of Elimination
Avapritinib is 70% eliminated in the feces with 11% as the unchanged drug and 18% eliminated in the urine with 0.23% as the unchanged drug.
Volume of Distribution
The mean apparent volume of distribution is 1200L.
Clearance
The mean apparent oral clearance of avapritinib is 19.5L/h.
Avapritinib is metabolized mainly by CYP3A4 and CYP2C9 in vitro. A 310mg oral dose is recovered as 49% unchanged drug, 35% hydroxy glucuronide metabolite, and 14% oxidatively deaminated metabolite.
The half life of avapritinib is 32-57h.
Avapritinib has a negative modulating effect on the transporters ABCB1 and ABCG2, which mediate the multidrug resistance phenotype of some cancers. This modulation may be due to interactions of avapritinib with the drug binding pocket of these transporters. Negative modulation of these transporters, resensitizes cancerous cells to treatment with chemotherapeutic agents like [paclitaxel].


