



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0

1. Ptc 124
2. Ptc-124
3. Ptc124
1. 775304-57-9
2. Ptc124
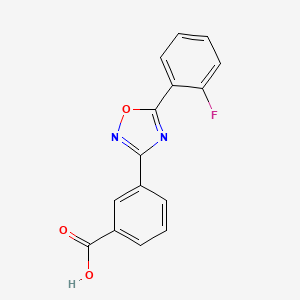
3. 3-(5-(2-fluorophenyl)-1,2,4-oxadiazol-3-yl)benzoic Acid
4. 3-[5-(2-fluorophenyl)-1,2,4-oxadiazol-3-yl]benzoic Acid
5. Ptc-124
6. Ataluren (ptc124)
7. Translarna
8. Ptc124 (ataluren)
9. Ptc 124
10. Benzoic Acid, 3-[5-(2-fluorophenyl)-1,2,4-oxadiazol-3-yl]-
11. K16ame9i3v
12. Ncgc00168759-02
13. Benzoic Acid, 3-(5-(2-fluorophenyl)-1,2,4-oxadiazol-3-yl)-
14. Dsstox_cid_26776
15. Dsstox_rid_81895
16. Dsstox_gsid_46776
17. Ataluren [usan]
18. Cas-775304-57-9
19. Ataluren [usan:inn]
20. Unii-k16ame9i3v
21. Translarna (tn)
22. Ptc124,ataluren
23. Ataluren(ptc124)
24. Ataluren; Ptc124
25. Ptc124 - Ataluren
26. Ataluren (usan/inn)
27. Ataluren [inn]
28. Ataluren [mi]
29. Ec-000.2051
30. Ataluren [who-dd]
31. Schembl60614
32. Mls006011160
33. Chembl256997
34. Gtpl7341
35. Dtxsid5046776
36. Chebi:94805
37. Ex-a385
38. Bcpp000097
39. Hms3656h20
40. Albb-036381
41. Bcp01756
42. Tox21_112631
43. Bbl102158
44. Mfcd09864996
45. Ncgc00168759
46. S6003
47. Stl555957
48. Zinc13831791
49. Akos005146455
50. Tox21_112631_1
51. Ccg-267286
52. Cs-0503
53. Db05016
54. Ex-3387
55. Gs-3946
56. Sb16685
57. Ncgc00168759-04
58. Ncgc00168759-05
59. Ncgc00168759-10
60. Ac-28390
61. Am808091
62. Hy-14832
63. Smr004702931
64. Bb 0261439
65. Ft-0651455
66. Sw219696-1
67. D09323
68. 304f579
69. Au-004/43508117
70. Q753330
71. Brd-k94830329-001-01-4
72. 3-[5-(2-fluoro-phenyl)-[1,2,4]oxadiazol-3-yl]-benzoic Acid
73. 3-[5-(2-fluorophenyl)-[1,2,4]oxadiazol-3-yl]benzoic Acid
74. Ptc124;3-[5-(2-fluorophenyl)-1,2,4-oxadiazol-3-yl]benzoic Acid;ptc-124
75. Jbf
| Molecular Weight | 284.24 g/mol |
|---|---|
| Molecular Formula | C15H9FN2O3 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 284.05972032 g/mol |
| Monoisotopic Mass | 284.05972032 g/mol |
| Topological Polar Surface Area | 76.2 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 382 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Ataluren is approved for use by the European Medicines Agency to treat Duchenne Muscular Dystrophy in patients aged 5 years and older who are able to walk. More specifically, ataluren is used in the small group of patients whose disease is caused by a specific genetic defect (called a nonsense mutation) in the dystrophin gene.
Translarna is indicated for the treatment of Duchenne muscular dystrophy resulting from a nonsense mutation in the dystrophin gene, in ambulatory patients aged 2 years and older. Efficacy has not been demonstrated in non-ambulatory patients.
The presence of a nonsense mutation in the dystrophin gene should be determined by genetic testing.
Treatment of cystic fibrosis
Treatment of dystrophinopathy
M09AX03
M - Musculo-skeletal system
M09 - Other drugs for disorders of the musculo-skeletal system
M09A - Other drugs for disorders of the musculo-skeletal system
M09AX - Other drugs for disorders of the musculo-skeletal system
M09AX03 - Ataluren
Absorption
Peak plasma levels of ataluren are attained approximately 1.5 hours after dosing in subjects who received medicinal product within 30 minutes of a meal.
Route of Elimination
After a single oral dose of radiolabeled ataluren, approximately half of the administered radioactive dose is recovered in the faeces and the remainder was recovered in the urine. In the urine, unchanged ataluren and the acyl glucuronide metabolite account for less than 1% and 49%, respectively, of the administered dose.
Ataluren is metabolized by conjugation via uridine diphosphate glucuronosyltransferase (UGT) enzymes, predominantly UGT1A9 in liver and intestine. In vivo, the only metabolite detected in plasma after oral administration of radio-labelled ataluren was the ataluren-O-1-acyl glucuronide; exposure to this metabolite in humans was approximately 8% of the plasma AUC of ataluren.
Ataluren plasma half-life ranges from 2-6 hours and is unaffected either by dose or repeated administration.
Ataluren enables ribosomal readthrough of mRNA containing premature stop codons that otherwise would result in premature termination of protein chains. Use of ataluren allows cellular machinery to bypass nonsense mutations in genetic material, continue the translation process, and thereby restore the production of a full-length, functional protein. The research on the effects of Ataluren on the translation and stability of nonsense-containing mRNA in vitor show that Ataluren promoted readthrough at each of the nonsense codons, showing maximal activity with UGA, while having no effect on mRNA levels. Unlike the stable cell line assays, Ataluren did not discriminate significantly between the UAG and UAA mRNAs. Ataluren was a more potent nonsense-suppressing agent than gentamicin, and exhibited 4- to 15-fold stimulation of in vitro readthrough relative to the controls at levels similar to those in the stable cell reporter assays. These results indicate that Ataluren modulates termination efficiency at premature nonsense codons.


