
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Ptc 124
2. Ptc-124
3. Ptc124
1. 775304-57-9
2. Ptc124
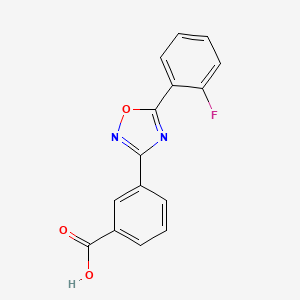
3. 3-(5-(2-fluorophenyl)-1,2,4-oxadiazol-3-yl)benzoic Acid
4. 3-[5-(2-fluorophenyl)-1,2,4-oxadiazol-3-yl]benzoic Acid
5. Ptc-124
6. Ataluren (ptc124)
7. Translarna
8. Ptc124 (ataluren)
9. Ptc 124
10. Benzoic Acid, 3-[5-(2-fluorophenyl)-1,2,4-oxadiazol-3-yl]-
11. K16ame9i3v
12. Ncgc00168759-02
13. Benzoic Acid, 3-(5-(2-fluorophenyl)-1,2,4-oxadiazol-3-yl)-
14. Dsstox_cid_26776
15. Dsstox_rid_81895
16. Dsstox_gsid_46776
17. Ataluren [usan]
18. Cas-775304-57-9
19. Ataluren [usan:inn]
20. Unii-k16ame9i3v
21. Translarna (tn)
22. Ptc124,ataluren
23. Ataluren(ptc124)
24. Ataluren; Ptc124
25. Ptc124 - Ataluren
26. Ataluren (usan/inn)
27. Ataluren [inn]
28. Ataluren [mi]
29. Ec-000.2051
30. Ataluren [who-dd]
31. Schembl60614
32. Mls006011160
33. Chembl256997
34. Gtpl7341
35. Dtxsid5046776
36. Chebi:94805
37. Ex-a385
38. Bcpp000097
39. Hms3656h20
40. Albb-036381
41. Bcp01756
42. Tox21_112631
43. Bbl102158
44. Mfcd09864996
45. Ncgc00168759
46. S6003
47. Stl555957
48. Zinc13831791
49. Akos005146455
50. Tox21_112631_1
51. Ccg-267286
52. Cs-0503
53. Db05016
54. Ex-3387
55. Gs-3946
56. Sb16685
57. Ncgc00168759-04
58. Ncgc00168759-05
59. Ncgc00168759-10
60. Ac-28390
61. Am808091
62. Hy-14832
63. Smr004702931
64. Bb 0261439
65. Ft-0651455
66. Sw219696-1
67. D09323
68. 304f579
69. Au-004/43508117
70. Q753330
71. Brd-k94830329-001-01-4
72. 3-[5-(2-fluoro-phenyl)-[1,2,4]oxadiazol-3-yl]-benzoic Acid
73. 3-[5-(2-fluorophenyl)-[1,2,4]oxadiazol-3-yl]benzoic Acid
74. Ptc124;3-[5-(2-fluorophenyl)-1,2,4-oxadiazol-3-yl]benzoic Acid;ptc-124
75. Jbf
| Molecular Weight | 284.24 g/mol |
|---|---|
| Molecular Formula | C15H9FN2O3 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 284.05972032 g/mol |
| Monoisotopic Mass | 284.05972032 g/mol |
| Topological Polar Surface Area | 76.2 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 382 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Ataluren is approved for use by the European Medicines Agency to treat Duchenne Muscular Dystrophy in patients aged 5 years and older who are able to walk. More specifically, ataluren is used in the small group of patients whose disease is caused by a specific genetic defect (called a nonsense mutation) in the dystrophin gene.
Translarna is indicated for the treatment of Duchenne muscular dystrophy resulting from a nonsense mutation in the dystrophin gene, in ambulatory patients aged 2 years and older. Efficacy has not been demonstrated in non-ambulatory patients.
The presence of a nonsense mutation in the dystrophin gene should be determined by genetic testing.
Treatment of cystic fibrosis
Treatment of dystrophinopathy
M09AX03
M - Musculo-skeletal system
M09 - Other drugs for disorders of the musculo-skeletal system
M09A - Other drugs for disorders of the musculo-skeletal system
M09AX - Other drugs for disorders of the musculo-skeletal system
M09AX03 - Ataluren
Absorption
Peak plasma levels of ataluren are attained approximately 1.5 hours after dosing in subjects who received medicinal product within 30 minutes of a meal.
Route of Elimination
After a single oral dose of radiolabeled ataluren, approximately half of the administered radioactive dose is recovered in the faeces and the remainder was recovered in the urine. In the urine, unchanged ataluren and the acyl glucuronide metabolite account for less than 1% and 49%, respectively, of the administered dose.
Ataluren is metabolized by conjugation via uridine diphosphate glucuronosyltransferase (UGT) enzymes, predominantly UGT1A9 in liver and intestine. In vivo, the only metabolite detected in plasma after oral administration of radio-labelled ataluren was the ataluren-O-1-acyl glucuronide; exposure to this metabolite in humans was approximately 8% of the plasma AUC of ataluren.
Ataluren plasma half-life ranges from 2-6 hours and is unaffected either by dose or repeated administration.
Ataluren enables ribosomal readthrough of mRNA containing premature stop codons that otherwise would result in premature termination of protein chains. Use of ataluren allows cellular machinery to bypass nonsense mutations in genetic material, continue the translation process, and thereby restore the production of a full-length, functional protein. The research on the effects of Ataluren on the translation and stability of nonsense-containing mRNA in vitor show that Ataluren promoted readthrough at each of the nonsense codons, showing maximal activity with UGA, while having no effect on mRNA levels. Unlike the stable cell line assays, Ataluren did not discriminate significantly between the UAG and UAA mRNAs. Ataluren was a more potent nonsense-suppressing agent than gentamicin, and exhibited 4- to 15-fold stimulation of in vitro readthrough relative to the controls at levels similar to those in the stable cell reporter assays. These results indicate that Ataluren modulates termination efficiency at premature nonsense codons.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

ABOUT THIS PAGE
76
PharmaCompass offers a list of Ataluren API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ataluren manufacturer or Ataluren supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ataluren manufacturer or Ataluren supplier.
PharmaCompass also assists you with knowing the Ataluren API Price utilized in the formulation of products. Ataluren API Price is not always fixed or binding as the Ataluren Price is obtained through a variety of data sources. The Ataluren Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ataluren manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ataluren, including repackagers and relabelers. The FDA regulates Ataluren manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ataluren API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ataluren manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ataluren supplier is an individual or a company that provides Ataluren active pharmaceutical ingredient (API) or Ataluren finished formulations upon request. The Ataluren suppliers may include Ataluren API manufacturers, exporters, distributors and traders.
click here to find a list of Ataluren suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Ataluren DMF (Drug Master File) is a document detailing the whole manufacturing process of Ataluren active pharmaceutical ingredient (API) in detail. Different forms of Ataluren DMFs exist exist since differing nations have different regulations, such as Ataluren USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Ataluren DMF submitted to regulatory agencies in the US is known as a USDMF. Ataluren USDMF includes data on Ataluren's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Ataluren USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Ataluren suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Ataluren as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Ataluren API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Ataluren as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Ataluren and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Ataluren NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Ataluren suppliers with NDC on PharmaCompass.
Ataluren Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ataluren GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ataluren GMP manufacturer or Ataluren GMP API supplier for your needs.
A Ataluren CoA (Certificate of Analysis) is a formal document that attests to Ataluren's compliance with Ataluren specifications and serves as a tool for batch-level quality control.
Ataluren CoA mostly includes findings from lab analyses of a specific batch. For each Ataluren CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ataluren may be tested according to a variety of international standards, such as European Pharmacopoeia (Ataluren EP), Ataluren JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ataluren USP).

