
Synopsis
Synopsis
0
EU WC
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
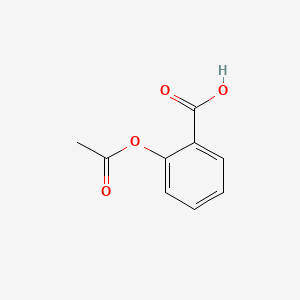

1. 2-(acetyloxy)benzoic Acid
2. Acetylsalicylic Acid
3. Acetysal
4. Acid, Acetylsalicylic
5. Acylpyrin
6. Aloxiprimum
7. Colfarit
8. Dispril
9. Easprin
10. Ecotrin
11. Endosprin
12. Magnecyl
13. Micristin
14. Polopirin
15. Polopiryna
16. Solprin
17. Solupsan
18. Zorprin
1. Acetylsalicylic Acid
2. 50-78-2
3. 2-acetoxybenzoic Acid
4. 2-(acetyloxy)benzoic Acid
5. O-acetylsalicylic Acid
6. Acetylsalicylate
7. O-acetoxybenzoic Acid
8. Acylpyrin
9. Easprin
10. Ecotrin
11. Salicylic Acid Acetate
12. Acenterine
13. Acetophen
14. Polopiryna
15. Acetosal
16. Colfarit
17. O-carboxyphenyl Acetate
18. Acidum Acetylsalicylicum
19. Enterosarein
20. Aceticyl
21. Acetonyl
22. Acetosalin
23. Acetylin
24. Aspergum
25. Aspirdrops
26. Benaspir
27. Measurin
28. Micristin
29. Pharmacin
30. Premaspin
31. Salcetogen
32. Temperal
33. Ecolen
34. Empirin
35. Endydol
36. Rhodine
37. Saletin
38. Rheumintabletten
39. Solprin Acid
40. 2-acetyloxybenzoic Acid
41. Benzoic Acid, 2-(acetyloxy)-
42. Acetisal
43. Acetylsal
44. Aspirine
45. Bialpirina
46. Bialpirinia
47. Claradin
48. Clariprin
49. Entericin
50. Enterophen
51. Enterosarine
52. Globentyl
53. Neuronika
54. Salacetin
55. Solpyron
56. Acesal
57. Acisal
58. Asagran
59. Asteric
60. Cemirit
61. Decaten
62. Duramax
63. Extren
64. Globoid
65. Helicon
66. Idragin
67. Levius
68. Pirseal
69. Rhonal
70. Solfrin
71. Adiro
72. Aspec
73. Aspro
74. Novid
75. Yasta
76. Acetosalic Acid
77. Triple-sal
78. Spira-dine
79. Zorprin
80. Bi-prin
81. Acetilum Acidulatum
82. Acimetten
83. Delgesic
84. Entrophen
85. Persistin
86. 2-carboxyphenyl Acetate
87. Acetilsalicilico
88. Dolean Ph 8
89. A.s.a. Empirin
90. Xaxa
91. Acido Acetilsalicilico
92. Contrheuma Retard
93. Acide Acetylsalicylique
94. Asa
95. Endosprin
96. Kapsazal
97. Bayer
98. Acetylsalicylsaure
99. Aspirin (acetylsalicylic Acid)
100. Solprin
101. Triaminicin
102. Asatard
103. Durlaza
104. Tasprin
105. Nu-seals Aspirin
106. Salicylic Acid, Acetate
107. Acido O-acetil-benzoico
108. Kyselina Acetylsalicylova
109. 2-acetoxybenzenecarboxylic Acid
110. St. Joseph Aspirin For Adults
111. A.s.a.
112. St. Joseph
113. Kyselina 2-acetoxybenzoova
114. Sp 189
115. Acetard
116. Ac 5230
117. Acetyl Salicylate
118. Acetylsalicylsaeure
119. Azetylsalizylsaeure
120. S-211
121. Ecm
122. Chebi:15365
123. 2-(acetyloxy)benzoate
124. Acetylsalicylicum Acidum
125. Nsc-27223
126. O-(acetyloxy)benzoic Acid
127. Nsc-406186
128. Acide 2-(acetyloxy)benzoique
129. Acetylsalicylic Acid (who-ip)
130. R16co5y76e
131. Aspirin Form Ii
132. Component Of Midol
133. Nsc27223
134. Component Of Synirin
135. 8-hour Bayer
136. Component Of Zactirin
137. Bay1019036
138. Component Of Coricidin
139. Component Of Persistin
140. Component Of Robaxisal
141. O-acetoxybenzoate
142. Ncgc00015067-04
143. Acetysal
144. Istopirin
145. Magnecyl
146. Medisyl
147. Polopirin
148. Ronal
149. Bayer Buffered
150. Dsstox_cid_108
151. Aspro Clear
152. Component Of Ascodeen-30
153. Bayer Plus
154. Wln: Qvr Bov1
155. Rheumin Tabletten
156. Acetylsalicylicacid
157. Dsstox_rid_75372
158. Dsstox_gsid_20108
159. Aspirina 03
160. Acetylsalycilic Acid
161. Acetyl Salicylic Acid
162. Component Of Darvon With A.s.a
163. Bayer Aspirin 8 Hour
164. Asaphen
165. Aspalon
166. Asprin
167. Bayer Children's Aspirin
168. Nu-seals
169. Component Of St. Joseph Cold Tablets
170. Aspir-mox
171. Durlaza Er
172. Acetylsalicylsaure [german]
173. Cas-50-78-2
174. Acetoxybenzoic Acid
175. Acetysalicylic Acid
176. 11126-35-5
177. Ain
178. Smr000059138
179. Ascoden-30
180. Benzoicacid, 2-(acetyloxy)-
181. Acetylsalicyclic Acid
182. Ccris 3243
183. Hsdb 652
184. Acide Acetylsalicylique [french]
185. Acido Acetilsalicilico [italian]
186. Kyselina Acetylsalicylova [czech]
187. Acido O-acetil-benzoico [italian]
188. Sr-01000075668
189. Kyselina 2-acetoxybenzoova [czech]
190. Bayer Extra Strength Aspirin For Migraine Pain
191. Einecs 200-064-1
192. Nsc 27223
193. Aspirin [usp:ban:jan]
194. Bayer Enteric 325 Mg Regular Strength
195. Brn 0779271
196. Bay E4465
197. Unii-r16co5y76e
198. Aspropharm
199. Bayer Enteric 81 Mg Adult Low Strength
200. Cardioaspirin
201. Cardioaspirina
202. Acetyonyl
203. Asacard
204. Ascolong
205. Bayer Enteric 500 Mg Arthritis Strength
206. Colsprin
207. Miniasal
208. Salospir
209. Acesan
210. Toldex
211. Ai3-02956
212. 1oxr
213. 2-acetoxybenzoate
214. Aspirin,(s)
215. Aspalon (jan)
216. Durlaza (tn)
217. Easprin (tn)
218. Mfcd00002430
219. Acetyl-salicylic Acid
220. Aspirin Usp-26
221. Acetyl Salicyclic Acid
222. O-(acetyloxy)benzoate
223. Percodan (salt/mix)
224. Ascriptin (salt/mix)
225. Micrainin (salt/mix)
226. 2-acetoxy Benzoic Acid
227. Spectrum_001245
228. 2-acetylsalicyclic Acid
229. Aspirin [vandf]
230. Aspirin [hsdb]
231. Salicylic Acid, Acetyl-
232. Aspirin [jan]
233. Aspirin [mi]
234. Chembl25
235. Aspirin [mart.]
236. Spectrum2_001899
237. Spectrum3_001295
238. Spectrum4_000099
239. Spectrum5_000740
240. Aspirin (jp17/usp)
241. Lopac-a-5376
242. Salycylacetylsalicylic Acid
243. Aspirin [usp-rs]
244. Benzoic Acid, 2-acetoxy-
245. Epitope Id:114151
246. Percodan Demi (salt/mix)
247. Soma Compound (salt/mix)
248. Zinc53
249. Ec 200-064-1
250. Acetylsalicylic Acid, 99%
251. Aspirin Usp (3080)
252. Cid_2244
253. Pravigard Pac (salt/mix)
254. Schembl1353
255. 2-(acetyloxy)-benzoic Acid
256. Aspirin Usp (2080b)
257. Bay-e-4465
258. Acetylsalicylic Acid-[13c]
259. Lopac0_000038
260. Kbiogr_000398
261. Kbiogr_002271
262. Kbioss_001725
263. Kbioss_002272
264. 4-10-00-00138 (beilstein Handbook Reference)
265. Mls001055329
266. Mls001066332
267. Mls001336045
268. Mls001336046
269. Aspirin [orange Book]
270. Bidd:gt0118
271. Divk1c_000555
272. Spectrum1500130
273. Spbio_001838
274. Acetylsalicylic Acid, >=99%
275. Axotal Component Aspirin
276. Azdone Component Aspirin
277. Codoxy Component Aspirin
278. Gtpl4139
279. (non-d)acetylsalicylic Acid-d3
280. Aspirin [usp Monograph]
281. O-acetylsalicylic Acid; Aspirin
282. Dtxsid5020108
283. Acetylsalicylic Acid-carboxy-14c
284. Aggrenox Component Aspirin
285. Bdbm22360
286. Duocover Component Aspirin
287. Excedrin Component Aspirin
288. Fiorinal Component Aspirin
289. Hms501l17
290. Kbio1_000555
291. Kbio2_001725
292. Kbio2_002271
293. Kbio2_004293
294. Kbio2_004839
295. Kbio2_006861
296. Kbio2_007407
297. Kbio3_002149
298. Kbio3_002751
299. Norgesic Component Aspirin
300. Percodan Component Aspirin
301. Q-gesic Component Aspirin
302. Roxiprin Component Aspirin
303. Vicoprin Component Aspirin
304. Yosprala Component Aspirin
305. Empirin With Codeine (salt/mix)
306. Acetylsalicylic Acid, >=99.0%
307. Cmap_000006
308. Component Of Zactirin (salt/mix)
309. Duoplavin Component Aspirin
310. Equagesic Component Aspirin
311. Invagesic Component Aspirin
312. Lanorinal Component Aspirin
313. Micrainin Component Aspirin
314. Ninds_000555
315. Robaxisal Component Aspirin
316. Aspirin Component Of Axotal
317. Aspirin Component Of Azdone
318. Aspirin Component Of Codoxy
319. Hms1920e13
320. Hms2090g03
321. Hms2091k13
322. Hms2233l18
323. Hms3260g17
324. Hms3372n15
325. Hms3656n14
326. Hms3715p19
327. Hms3866l03
328. Hms3885g03
329. Pharmakon1600-01500130
330. Acetylsalicylic Acid [inci]
331. Bcp21790
332. Orphengesic Component Aspirin
333. Str01551
334. Acetylsalicylic Acid; Aspirin
335. Aspirin Component Of Aggrenox
336. Aspirin Component Of Duocover
337. Aspirin Component Of Excedrin
338. Aspirin Component Of Fiorinal
339. Aspirin Component Of Norgesic
340. Aspirin Component Of Percodan
341. Aspirin Component Of Q-gesic
342. Aspirin Component Of Roxiprin
343. Aspirin Component Of Vicoprin
344. Aspirin Component Of Yosprala
345. Synalgos-dc Component Aspirin
346. Tox21_110076
347. Tox21_202117
348. Tox21_300146
349. Tox21_500038
350. Ccg-39490
351. Nsc406186
352. Nsc755899
353. S3017
354. Stl137674
355. Acetylsalicylic Acid [who-dd]
356. Aspirin Component Of Duoplavin
357. Aspirin Component Of Equagesic
358. Aspirin Component Of Invagesic
359. Aspirin Component Of Lanorinal
360. Aspirin Component Of Micrainin
361. Aspirin Component Of Robaxisal
362. Akos000118884
363. Component Of Ascodeen-30 (salt/mix)
364. Mepro-aspirin Component Aspirin
365. Percodan-demi Component Aspirin
366. Pravigard Pac Component Aspirin
367. Soma Compound Component Aspirin
368. Tox21_110076_1
369. Acetylsalicylic Acid [ema Epar]
370. Acetylsalicylicum Acidum [hpus]
371. Aspirin Component Of Orphengesic
372. Cs-2001
373. Db00945
374. Lp00038
375. Nsc-755899
376. Pl-2200
377. Sdccgsbi-0050027.p005
378. Aspirin Component Of Synalgos-dc
379. Bay-1019036
380. Darvon Compound Component Aspirin
381. Idi1_000555
382. Invagesic Forte Component Aspirin
383. Talwin Compound Component Aspirin
384. Acetylsalicylic Acid [green Book]
385. Acetylsalicylic Acid, Analytical Standard
386. Acidum Acetylsalicylicum (who-ip)
387. Ncgc00015067-01
388. Ncgc00015067-02
389. Ncgc00015067-03
390. Ncgc00015067-05
391. Ncgc00015067-06
392. Ncgc00015067-07
393. Ncgc00015067-08
394. Ncgc00015067-09
395. Ncgc00015067-10
396. Ncgc00015067-11
397. Ncgc00015067-12
398. Ncgc00015067-13
399. Ncgc00015067-14
400. Ncgc00015067-24
401. Ncgc00015067-26
402. Ncgc00090977-01
403. Ncgc00090977-02
404. Ncgc00090977-03
405. Ncgc00090977-04
406. Ncgc00090977-05
407. Ncgc00090977-06
408. Ncgc00090977-07
409. Ncgc00254034-01
410. Ncgc00259666-01
411. Ncgc00260723-01
412. Aspirin Component Of Mepro-aspirin
413. Aspirin Component Of Percodan-demi
414. Aspirin Component Of Pravigard Pac
415. Aspirin Component Of Soma Compound
416. Aspirin, Meets Usp Testing Specifications
417. Hy-14654
418. Nci60_002222
419. Orphengesic Forte Component Aspirin
420. Acetylsalicylic Acid [ep Monograph]
421. Sbi-0050027.p004
422. Aspirin Component Of Darvon Compound
423. Aspirin Component Of Invagesic Forte
424. Aspirin Component Of Talwin Compound
425. Ds-017139
426. Unm-0000306102
427. Aspirin Component Of Orphengesic Forte
428. Component Of Darvon With A.s.a (salt/mix)
429. Eu-0100038
430. Ft-0655181
431. Ft-0661360
432. Sw199665-2
433. Carisoprodol Compound Component Aspirin
434. A 5376
435. Acetylsalicylic Acid 1.0 Mg/ml In Acetonitrile
436. C01405
437. D00109
438. E80792
439. Q18216
440. Ab00051918-08
441. Ab00051918_09
442. Ab00051918_10
443. Aspirin Component Of Carisoprodol Compound
444. Arthritis Pain Formula Maximum Strength (salt/mix)
445. Sr-01000075668-1
446. Sr-01000075668-4
447. Sr-01000075668-6
448. Acetylsalicylic Acid, Vetec(tm) Reagent Grade, >=99%
449. Aspirin, British Pharmacopoeia (bp) Reference Standard
450. Clopidogrel/acetylsalicylic Acid Component Aspirin
451. F2191-0068
452. Z234893989
453. Aspirin Component Of Clopidogrel/acetylsalicylic Acid
454. Aspirin, United States Pharmacopeia (usp) Reference Standard
455. D41527a7-a9eb-472d-a7fc-312821130549
456. Acetylsalicylic Acid, European Pharmacopoeia (ep) Reference Standard
457. Acetylsalicylic Acid, Bioreagent, Plant Cell Culture Tested, >=99.0%
458. Acetylsalicylic Acid For Peak Identification, European Pharmacopoeia (ep) Reference Standard
459. Aspirin (acetyl Salicylic Acid), Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 180.16 g/mol |
|---|---|
| Molecular Formula | C9H8O4 |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 180.04225873 g/mol |
| Monoisotopic Mass | 180.04225873 g/mol |
| Topological Polar Surface Area | 63.6 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 212 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Aspirin |
| PubMed Health | Aspirin |
| Drug Classes | Analgesic, Antipyretic, Antirheumatic, Central Nervous System Agent, Platelet Aggregation Inhibitor |
| Drug Label | Distributed by GENUINE FIRST AID600 Clevelad Str Suite 400, Clearwater, FL 33755... |
| Active Ingredient | Aspirin |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 325mg |
| Market Status | Over the Counter |
| Company | Plx Pharma |
| 2 of 2 | |
|---|---|
| Drug Name | Aspirin |
| PubMed Health | Aspirin |
| Drug Classes | Analgesic, Antipyretic, Antirheumatic, Central Nervous System Agent, Platelet Aggregation Inhibitor |
| Drug Label | Distributed by GENUINE FIRST AID600 Clevelad Str Suite 400, Clearwater, FL 33755... |
| Active Ingredient | Aspirin |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 325mg |
| Market Status | Over the Counter |
| Company | Plx Pharma |
Anti-Inflammatory Agents, Non-Steroidal; Cyclooxygenase Inhibitors; Fibrinolytic Agents; Platelet Aggregation Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Salicylates are indicated to relieve myalgia, musculoskeletal pain, and other symptoms of nonrheumatic inflammatory conditions such as athletic injuries, bursitis, capsulitis, tendinitis, and nonspecific acute tenosynovitis. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2574
Salicylates are indicated for the symptomatic relief of acute and chronic rheumatoid arthritis, juvenile arthritis, osteoarthritis, and related rheumatic diseases. Aspirin is usually the first agent to be used and may be the drug of choice in patients able to tolerate prolonged therapy with high doses. These agents do not affect the progressive course of rheumatoid arthritis. Concurrent treatment with a glucocorticoid or a disease-modifying antirheumatic agent may be needed, depending on the condition being treated and patient response. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2574
Salicylates are also used to reduce arthritic complications associated with systemic lupus erythematosus. /Salicylates; NOT included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2574
For more Therapeutic Uses (Complete) data for ACETYLSALICYLIC ACID (12 total), please visit the HSDB record page.
Aspirin use may be associated with the development of Reye's syndrome in children and teenagers with acute febrile illnesses, especially influenza and varicella. It is recommended that salicylate therapy not be initiated in febrile pediatric or adolescent patients until after the presence of such an illness has been ruled out. Also, it is recommended that chronic salicylate therapy in these patients be discontinued if a fever occurs, and not resumed until it has been determined that an illness that may predispose to Reye's syndrome is not present or has run its course. Other forms of salicylate toxicity may also be more prevalent in pediatric patients, especially children who have a fever or are dehydrated.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2577
Especially careful monitoring of the serum salicylate concentration is recommended in pediatric patients with Kawasaki disease. Absorption of aspirin is impaired during the early febrile stage of the disease; therapeutic anti-inflammatory plasma salicylate concentrations may be extremely difficult to achieve. Also, as the febrile stage passes, absorption is improved; salicylate toxicity may occur if dosage is not readjusted.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2577
Requirements of Vitamin K may be increased in patients receiving high doses of salicylate. /Salicylate/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2578
IF RENAL FUNCTION IS COMPROMISED IN SALICYLATE INTOXICATION, POTASSIUM LOST FROM CELLS ACCUMULATES IN EXTRACELLULAR FLUID & POTASSIUM INTOXICATION MAY OCCUR.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 650
For more Drug Warnings (Complete) data for ACETYLSALICYLIC ACID (21 total), please visit the HSDB record page.
The lethal dose of aspirin for an adult is probably in the region of 25 to 30 g but recovery has been achieved by appropriate treatment after the ingestion of twice or thrice this amount.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 236
**Pain, fever, and inflammation** Acetylsalicylic acid (ASA), in the regular tablet form (immediate-release), is indicated to relieve pain, fever, and inflammation associated with many conditions, including the flu, the common cold, neck and back pain, dysmenorrhea, headache, tooth pain, sprains, fractures, myositis, neuralgia, synovitis, arthritis, bursitis, burns, and various injuries. It is also used for symptomatic pain relief after surgical and dental procedures. The _extra strength_ formulation of acetylsalicylic acid is also indicated for the management migraine pain with photophobia (sensitivity to light) and phonophobia (sensitivity to sound). **Other indications** ASA is also indicated for various other purposes, due to its ability to inhibit platelet aggregation. These include: Reducing the risk of cardiovascular death in suspected cases of myocardial infarction (MI). Reducing the risk of a first non-fatal myocardial infarction in patients, and for reducing the risk of morbidity and mortality in cases of unstable angina and in those who have had a prior myocardial infarction. For reducing the risk of transient ischemic attacks (TIA) and to prevent atherothrombotic cerebral infarction (in conjunction with other treatments). For the prevention of thromboembolism after hip replacement surgery. For decreasing platelet to platelet adhesion following carotid endarterectomy, aiding in the prevention of transient ischemic attacks (TIA). Used for patients undergoing hemodialysis with a silicone rubber arteriovenous cannula inserted to prevent thrombosis at the insertion site. **Important note regarding use of the extended-release formulation** In the setting of acute myocardial infarction, or before percutaneous interventions, the extended-release form of acetylsalicylic acid should not be used. Use immediate-release formulations in scenarios requiring rapid onset of action. The extended-release form is taken to decrease the incidence of mortality and myocardial infarction (MI) for individuals diagnosed with chronic coronary artery disease (CAD), including patients with previous myocardial infarction (MI) or unstable angina or with chronic stable angina. Additionally, the extended-release form is used to decrease the risk of death and recurrent episodes of stroke in patients with a history of stroke or TIA.
FDA Label
**Effects on pain and fever** Acetylsalicylic acid disrupts the production of prostaglandins throughout the body by targeting cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2). Prostaglandins are potent, irritating substances that have been shown to cause headaches and pain upon injection into humans. Prostaglandins increase the sensitivity of pain receptors and substances such as histamine and bradykinin. Through the disruption of the production and prevention of release of prostaglandins in inflammation, this drug may stop their action at pain receptors, preventing symptoms of pain. Acetylsalicylic acid is considered an antipyretic agent because of its ability to interfere with the production of brain prostaglandin E1. Prostaglandin E1 is known to be an extremely powerful fever-inducing agent. **Effects on platelet aggregation** The inhibition of platelet aggregation by ASA occurs because of its interference with thromboxane A2 in platelets, caused by COX-1 inhibition. Thromboxane A2 is an important lipid responsible for platelet aggregation, which can lead to clot formation and future risk of heart attack or stroke. **A note on cancer prevention** ASA has been studied in recent years to determine its effect on the prevention of various malignancies. In general, acetylsalicylic acid is involved in the interference of various cancer signaling pathways, sometimes inducing or upregulating tumor suppressor genes. Results of various studies suggest that there are beneficial effects of long-term ASA use in the prevention of several types of cancer, including stomach, colorectal, pancreatic, and liver cancers. Research is ongoing.
Antipyretics
Drugs that are used to reduce body temperature in fever. (See all compounds classified as Antipyretics.)
Cyclooxygenase Inhibitors
Compounds or agents that combine with cyclooxygenase (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASES) and thereby prevent its substrate-enzyme combination with arachidonic acid and the formation of eicosanoids, prostaglandins, and thromboxanes. (See all compounds classified as Cyclooxygenase Inhibitors.)
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Fibrinolytic Agents
Fibrinolysin or agents that convert plasminogen to FIBRINOLYSIN. (See all compounds classified as Fibrinolytic Agents.)
N02BA01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A01 - Stomatological preparations
A01A - Stomatological preparations
A01AD - Other agents for local oral treatment
A01AD05 - Acetylsalicylic acid
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AC - Platelet aggregation inhibitors excl. heparin
B01AC06 - Acetylsalicylic acid
N - Nervous system
N02 - Analgesics
N02B - Other analgesics and antipyretics
N02BA - Salicylic acid and derivatives
N02BA01 - Acetylsalicylic acid
Absorption
Absorption is generally rapid and complete following oral administration but absorption may be variable depending on the route, dosage form, and other factors including but not limited to the rate of tablet dissolution, gastric contents, gastric emptying time, and gastric pH. **Detailed absorption information** When ingested orally, acetylsalicylic acid is rapidly absorbed in both the stomach and proximal small intestine. The non-ionized acetylsalicylic acid passes through the stomach lining by passive diffusion. Ideal absorption of salicylate in the stomach occurs in the pH range of 2.15 - 4.10. Intestinal absorption of acetylsalicylic acid occurs at a much faster rate. At least half of the ingested dose is hydrolyzed to salicylic acid in the first-hour post-ingestion by esterases found in the gastrointestinal tract. Peak plasma salicylate concentrations occur between 1-2 hours post-administration.
Route of Elimination
Excretion of salicylates occurs mainly through the kidney, by the processes of glomerular filtration and tubular excretion, in the form of free salicylic acid, salicyluric acid, and, additionally, phenolic and acyl glucuronides. Salicylate can be found in the urine soon after administration, however, the entire dose takes about 48 hours to be completely eliminated. The rate of salicylate is often variable, ranging from 10% to 85% in the urine, and heavily depends on urinary pH. Acidic urine generally aids in reabsorption of salicylate by the renal tubules, while alkaline urine increases excretion. After the administration of a typical 325mg dose, the elimination of ASA is found to follow first order kinetics in a linear fashion. At high concentrations, the elimination half-life increases.
Volume of Distribution
This drug is distributed to body tissues shortly after administration. It is known to cross the placenta. The plasma contains high levels of salicylate, as well as tissues such as spinal, peritoneal and synovial fluids, saliva and milk. The kidney, liver, heart, and lungs are also found to be rich in salicylate concentration after dosing. Low concentrations of salicylate are usually low, and minimal concentrations are found in feces, bile, and sweat.
Clearance
The clearance rate of acetylsalicylic acid is extremely variable, depending on several factors. Dosage adjustments may be required in patients with renal impairment. The extended-release tablet should not be administered to patients with eGFR of less than 10 mL/min.
The materno-fetal transfer of salicylic acid and its distribution in the fetal organism was investigated in women of early pregnancy. Acetylsalicylic acid was administered orally in a single dose or in repeated doses at different times before legal interruption. The mean passage rates were about 6-15%. They were independent of the maternal serum concentrations of salicylic acid. The distribution of salicylic acid on the fetal liver, intestine, kidneys, lungs and brain was different. All fetal organs (9th to 15th week of gestation) studied exhibit an acetylsalicylic acid-splitting esterase activity. The esterase activity of the fetal liver was about 30% of the hydrolytic activity of the adult liver. The esterase activity was mainly located in the 105 000 X g-supernatant of cell homogenates.
PMID:6651815 Amon I et al; Biomed Biochim Acta 42 (7-8): 997-1004 (1983)
Approximately 80-100% of an oral dose of aspirin is absorbed from the GI tract. However, the actual bioavailability of the drug as unhydrolyzed aspirin is lower since aspirin is partially hydrolyzed to salicylate in the GI mucosa during absorption and on first pass through the liver. There are relatively few studies of the bioavailability of unhydrolyzed aspirin. In one study in which aspirin was administered IV and as an oral aqueous solution, it was shown that the solution was completely absorbed but only about 70% reached the systemic circulation as unhydrolyzed aspirin. In another study in which aspirin was administered IV and orally as capsules, only about 50% of the oral dose reached the systemic circulation as unhydrolyzed aspirin. There is some evidence that the bioavailability of unhydrolyzed aspirin from slowly absorbed dosage forms (e.g., enteric-coated tablets) may be substantially decreased. Food does not appear to decrease the bioavailability of unhydrolyzed aspirin or salicylate; however, absorption is delayed and peak serum aspirin or salicylate concentration may be decreased. There is some evidence that absorption of salicylate following oral administration may be substantially impaired or is highly variable during the febrile phase of Kawasaki disease.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2036
A 52 year-old woman ingested approximately 300 tablets (325 mg) of aspirin in a suicide attempt. ... The concentrations of salicylic acid in heart and femoral blood were 1.1 mg/mL and 1.3 mg/mL, respectively; the results were far higher than the lethal level. The concentration of salicylic acid was 0.3-0.4 mg/g in brain, 0.9-1.4 mg/g in lung, 0.6-0.8 mg/g in liver and 0.9 mg/mL in kidney.
PMID:18044220 Ihama Y t al; Chudoku Kenkyu 20 (4): 375-80 (2007)
The study was undertaken to determine the distribution of aspirin and its metabolites in the semen of humans after an oral dose of aspirin. Each of seven healthy male volunteers was given a single oral dose of 975 mg of aspirin on an empty stomach together with 200 mL of water. Timed samples of blood and semen were obtained from each subject, and the concentrations of aspirin, salicylic acid, and salicyluric acid determined by a specific high-performance liquid chromatographic assay. The mean peak concentration of aspirin was 6.5 micrograms/mL in plasma (range, 4.9-8.9 micrograms/mL), reached in 26 minutes (range, 13-33 minutes). The half-life of aspirin was 31 minutes. The concentration ratio of aspirin (semen/plasma) was 0.12 (except for one subject in whom it was 0.025). The mean peak concentration of salicylate in plasma was 49 micrograms/mL (range, 42-62 micrograms/mL), reached in 2.5 hours (range, 2.0-2.8 hours). Salicylate distributed rapidly into semen and maintained a concentration ratio (semen/plasma) of 0.15. Salicyluric acid (the glycine conjugate of salicylic acid) was found in the semen. Its high concentration in some subjects' semen (four times the concurrent plasma concentration) was attributed to contamination of semen sample with residual urine, containing salicylurate, in the urethra of those who urinated after the dose of aspirin. Possible side effects of aspirin and salicylate in semen include adverse effects on fertility, male-medicated teratogenesis, dominant lethal mutations, and hypersensitivity reactions in the recipients.
PMID:3680588 Kershaw RA et al; J Clin Pharmacol 27 (4): 304-9 (1987)
For more Absorption, Distribution and Excretion (Complete) data for ACETYLSALICYLIC ACID (12 total), please visit the HSDB record page.
Acetylsalicylic acid is hydrolyzed in the plasma to salicylic acid. Plasma concentrations of aspirin following after administration of the extended-release form are mostly undetectable 4-8 hours after ingestion of a single dose. Salicylic acid was measured at 24 hours following a single dose of extended-release acetylsalicylic acid. Salicylate is mainly metabolized in the liver, although other tissues may also be involved in this process. The major metabolites of acetylsalicylic acid are salicylic acid, salicyluric acid, the ether or phenolic glucuronide and the ester or acyl glucuronide. A small portion is converted to gentisic acid and other hydroxybenzoic acids.
Acetylsalicylic acid is hydrolyzed in the stomach and in blood to salicylic acid and acetic acid; ... .
International Programme on Chemical Safety; Poisons Information Monograph: Acetylsalicylic Acid (PIM 006) (1991) Available from, as of March 10, 2008: https://www.inchem.org/pages/pims.html
MAJOR URINARY METABOLITES OF ASPIRIN INCL SALICYLURONIC ACID ... SALICYL-O-GLUCURONIDE ... & SALICYL ESTER GLUCURONIDE ... & FREE SALICYLIC ACID ... .
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 183
A 52 year-old woman ingested approximately 300 tablets (325 mg) of aspirin in a suicide attempt. /Investigators/ analyzed the concentrations of salicylic acid (SA) and salicyluric acid (SUA) in body fluids and organs using a modified previous high-performance liquid chromatographic method. The concentrations of SA in heart and femoral blood were 1.1 mg/mL and 1.3 mg/mL, respectively; the results were far higher than the lethal level. The concentration of SA was 0.3-0.4 mg/g in brain, 0.9-1.4 mg/g in lung, 0.6-0.8 mg/g in liver and 0.9 mg/mL in kidney.
PMID:18044220 Ihama Y et al; Chudoku Kenkyu 20 (4): 375-80 (2007)
The half-life of ASA in the circulation ranges from 13 - 19 minutes. Blood concentrations drop rapidly after complete absorption. The half-life of the salicylate ranges between 3.5 and 4.5 hours.
15 to 20 minutes (for intact molecule); rapidly hydrolyzed to salicylate. In breast milk (as salicylate): 3.8 to 12.5 hours (average 7.1 hours) following a single 650 mg dose of aspirin.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2576
Cats are deficient in glucuronyl transferase and have a prolonged excretion of aspirin (the half-life in cats is 37.5 hr).
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2528
Acetylsalicylic acid (ASA) blocks prostaglandin synthesis. It is non-selective for COX-1 and COX-2 enzymes. Inhibition of COX-1 results in the inhibition of platelet aggregation for about 7-10 days (average platelet lifespan). The acetyl group of acetylsalicylic acid binds with a serine residue of the cyclooxygenase-1 (COX-1) enzyme, leading to irreversible inhibition. This prevents the production of pain-causing prostaglandins. This process also stops the conversion of arachidonic acid to thromboxane A2 (TXA2), which is a potent inducer of platelet aggregation. Platelet aggregation can result in clots and harmful venous and arterial thromboembolism, leading to conditions such as pulmonary embolism and stroke. It is important to note that there is 60% homology between the protein structures of COX-1 and COX-2. ASA binds to serine 516 residue on the active site of COX-2 in the same fashion as its binding to the serine 530 residue located on the active site of COX-1. The active site of COX-2 is, however, slightly larger than the active site of COX-1, so that arachidonic acid (which later becomes prostaglandins) manages to bypass the aspirin molecule inactivating COX-2. ASA, therefore, exerts more action on the COX-1 receptor rather than on the COX-2 receptor. A higher dose of acetylsalicylic acid is required for COX-2 inhibition.
Produce analgesia through a peripheral action by blocking pain impulse generation and via a central action, possibly in the hypothalamus. The peripheral action may predominate and probably involves inhibition of the synthesis or prostaglandins, and possibly inhibition of the synthesis and/or actions of other substances, which sensitize pain receptors to mechanical or chemical stimulation. /Salicylates/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2575
May produce antipyresis by acting centrally on the hypothalamic heat-regulating center to produce peripheral vasodilation resulting in increased cutaneous blood flow, sweating, and heat loss. The central action may involve inhibition of prostaglandin synthesis in the hypothalamus; however, there is some evidence that fevers caused by endogenous pyrogens that do not act via a prostaglandin mechanism may also respond to salicylate therapy. /Salicylates/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2575
CNS ... ESP NUCLEI LOCATED IN HYPOTHALAMUS PLAYS MAJOR ROLE IN REGULATION OF PERIPHERAL MECHANISMS CONCERNED WITH BODY HEAT PRODN & LOSS. WITH SALICYLATES, HEAT PRODN IS NOT INHIBITED, BUT HEAT LOSS IS INCR BY INCR PERIPHERAL BLOOD FLOW & PERSPIRATION. /SALICYLATES/
Evaluations of Drug Interactions. 2nd ed. and supplements. Washington, DC: American Pharmaceutical Assn., 1976, 1978., p. 341
Aspirin acetylates prostaglandin endoperoxide synthase (prostaglandin G/H-synthase) and irreversibly inhibits its cyclooxygenase (COX) activity. The enzyme catalyzes the conversion of arachidonic acid to PGH2, the first committed step in prostanoid biosynthesis. Two isoforms of prostaglandin endoperoxide synthase exist, PGHS-1 and PGHS-2 (also referred to as COX-1 and COX-2, respectively). PGHS-1 (COX-1) is expressed constitutively in most cell types, including platelets. PGHS-2 (COX-2) is undetectable in most mammalian cells, but its expression can be induced rapidly in response to mitogenic and inflammatory stimuli. Aspirin is a relatively selective inhibitor of platelet PGHS-1 (cyclooxygenase-1, COX-1). The existence of 2 isoenzymes with different aspirin sensitivities, coupled with extremely different recovery rates of their cyclooxygenase (COX) activity following inactivation by aspirin, at least partially explains the different dosage requirements and durations of aspirin effects on platelet function versus the drug's analgesic and anti-inflammatory effects. Human platelets and vascular endothelial cells process PGH2 to produce thromboxane A2 and prostacyclin (epoprostenol, PGI2), respectively. Thromboxane A2 induces platelet aggregation and vasoconstriction, while prostacyclin inhibits platelet aggregation and induces vasodilation. Aspirin is antithrombotic in a wide range of doses inhibiting thromboxane A2 and prostacyclin.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2035
For more Mechanism of Action (Complete) data for ACETYLSALICYLIC ACID (12 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
66
PharmaCompass offers a list of Aspirin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Aspirin manufacturer or Aspirin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Aspirin manufacturer or Aspirin supplier.
PharmaCompass also assists you with knowing the Aspirin API Price utilized in the formulation of products. Aspirin API Price is not always fixed or binding as the Aspirin Price is obtained through a variety of data sources. The Aspirin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Aspro manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Aspro, including repackagers and relabelers. The FDA regulates Aspro manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Aspro API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Aspro manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Aspro supplier is an individual or a company that provides Aspro active pharmaceutical ingredient (API) or Aspro finished formulations upon request. The Aspro suppliers may include Aspro API manufacturers, exporters, distributors and traders.
click here to find a list of Aspro suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Aspro DMF (Drug Master File) is a document detailing the whole manufacturing process of Aspro active pharmaceutical ingredient (API) in detail. Different forms of Aspro DMFs exist exist since differing nations have different regulations, such as Aspro USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Aspro DMF submitted to regulatory agencies in the US is known as a USDMF. Aspro USDMF includes data on Aspro's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Aspro USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Aspro suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Aspro Drug Master File in Japan (Aspro JDMF) empowers Aspro API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Aspro JDMF during the approval evaluation for pharmaceutical products. At the time of Aspro JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Aspro suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Aspro Drug Master File in Korea (Aspro KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Aspro. The MFDS reviews the Aspro KDMF as part of the drug registration process and uses the information provided in the Aspro KDMF to evaluate the safety and efficacy of the drug.
After submitting a Aspro KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Aspro API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Aspro suppliers with KDMF on PharmaCompass.
A Aspro CEP of the European Pharmacopoeia monograph is often referred to as a Aspro Certificate of Suitability (COS). The purpose of a Aspro CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Aspro EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Aspro to their clients by showing that a Aspro CEP has been issued for it. The manufacturer submits a Aspro CEP (COS) as part of the market authorization procedure, and it takes on the role of a Aspro CEP holder for the record. Additionally, the data presented in the Aspro CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Aspro DMF.
A Aspro CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Aspro CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Aspro suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Aspro as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Aspro API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Aspro as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Aspro and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Aspro NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Aspro suppliers with NDC on PharmaCompass.
Aspro Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Aspro GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Aspro GMP manufacturer or Aspro GMP API supplier for your needs.
A Aspro CoA (Certificate of Analysis) is a formal document that attests to Aspro's compliance with Aspro specifications and serves as a tool for batch-level quality control.
Aspro CoA mostly includes findings from lab analyses of a specific batch. For each Aspro CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Aspro may be tested according to a variety of international standards, such as European Pharmacopoeia (Aspro EP), Aspro JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Aspro USP).

