
Synopsis
Synopsis
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
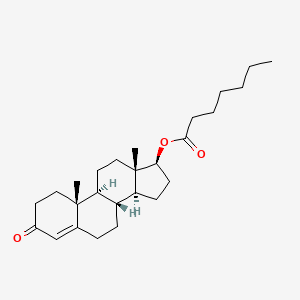

1. Andropository
2. Delatestryl
3. Durathate
4. Primoteston Depot
5. Testosteron Depot-rotexmedica
6. Testosteron-depot Eifelfango
7. Testosteron-depot Jenapharm
8. Testosterone Heptanoate
9. Testosterone Heptylate
10. Testrin P.a.
11. Theramex
1. 315-37-7
2. Testosterone Heptanoate
3. Androtardyl
4. Testosterone Enantate
5. Testosterone Heptylate
6. Atlatest
7. Testanthate
8. Testinon
9. Testoenant
10. Testostroval
11. Everone
12. Orquisteron-e
13. Exten Test
14. Depo-testro Med
15. Testosterone Oenanthate
16. Andropository
17. Durathate
18. Testenate
19. Testosterone Heptoate
20. Reposo-tmd
21. Primotestone
22. Malogen L.a.
23. Depatestrye
24. Testosterone 17-enanthate
25. Nsc-17591
26. Malogen L.a.200
27. Reposo Tmd
28. Testonenant
29. Ditate
30. Andro L.a. 200
31. 17-hydroxyandrost-4-en-3-one, 17-heptanoate
32. Delatest
33. Testate
34. Xyosted
35. Testosterone, Heptanoate
36. Heptanoic Acid, Ester With Testosterone
37. 17-((1-oxoheptyl)oxy)androst-4-en-3-one
38. 17beta-enanthoxyandrost-4-en-3-one
39. 4-androsten-3-one 17beta-enanthate
40. Androgyn L.a.
41. Androst-4-en-3-one, 17-[(1-oxoheptyl)oxy]-, (17b)-
42. Dea No. 4000
43. 17beta-hydroxyandrost-4-en-3-one Enanthate
44. Androst-4-en-3-one, 17-((1-oxoheptyl)oxy)-, (17beta)-
45. Testosterone Enanthate Ciii
46. Chebi:9464
47. 7z6522t8n9
48. [(8r,9s,10r,13s,14s,17s)-10,13-dimethyl-3-oxo-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-17-yl] Heptanoate
49. 17-heptanoyl-17beta-hydroxyandrost-4-en-3-one
50. (8r,9s,10r,13s,14s,17s)-10,13-dimethyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl Heptanoate
51. Testosterone Ethanate
52. Ccris 7082
53. 17-[(1-oxoheptyl)oxy]androst-4-en-3-one
54. Einecs 206-253-5
55. Brn 3170544
56. Androst-4-en-3-one, 17-[(1-oxoheptyl)oxy]-, (17.beta.)-
57. Unii-7z6522t8n9
58. Testosterone Enanthate [usp:jan]
59. Delatestryl (tn)
60. Androst-4-en-3-one, 17beta-hydroxy-, Heptanoate
61. Testosteroni Enantas
62. Ditate (salt/mix)
63. Androst-4-en-3-one, 17-(1-oxoheptyl)oxy-, (17beta)-
64. Deladumone (salt/mix)
65. Testosterone Heptoic Acid
66. Enanthic Acid Testosterone
67. Schembl42687
68. Androgyn L.a. (salt/mix)
69. 4-08-00-00979 (beilstein Handbook Reference)
70. Testosterone 17beta-heptanoate
71. Chembl1200335
72. Androst-4-en-3-one, Heptanoate
73. Dtxsid701016540
74. Testosterone 17beta-heptanoic Acid
75. Testosterone Enanthate [mi]
76. Nsc17591
77. Zinc3876080
78. Testosterone Enanthate (jp17/usp)
79. Testosterone Enanthate [jan]
80. Lmst02020075
81. S3717
82. Androst-4-en-3-one,(17.beta.)-
83. Testosterone Enantate [mart.]
84. Testosterone Enanthate [vandf]
85. Akos015960945
86. Testosterone Enantate [who-ip]
87. Ccg-268655
88. Db13944
89. Testosterone Enanthate [usp-rs]
90. Testosterone Enanthate [who-dd]
91. 4-androsten-3-one 17.beta.-enanthate
92. 3-oxoandrost-4-en-17beta-yl Heptanoate
93. Ac-12599
94. Ds-11585
95. Testosterone Enanthate, Analytical Standard
96. 4-androsten-17beta-ol-3-one 17-enanthate
97. Testosterone Enantate [ep Monograph]
98. Testosterone Enanthate [orange Book]
99. Testosteroni Enantas [who-ip Latin]
100. 17beta-hydroxyandrost-4-en-3-one Heptanoate
101. Testosterone Enanthate Ciii [usp-rs]
102. Testosterone Enanthate [usp Monograph]
103. Wln: L E5 B666 Ov Mutj A E Fov6
104. C08157
105. D00958
106. Ditate-ds Component Testosterone Enanthate
107. 17-hydroxyandrost-4-en-3-one, 17-heptanoic Acid
108. 315t377
109. Sr-01000942262
110. Sr-01000942262-1
111. Testosterone Enanthate Component Of Ditate-ds
112. W-106891
113. Androst-4-en-3-one, 17.beta.-hydroxy-, Heptanoate
114. Q27108402
115. 3-oxoandrost-4-en-17-yl Heptanoate, (17.beta.)- #
116. 17.beta.-(heptanoyloxy)androst-4-en-3-one [who-ip]
117. Androst-4-en-3-one, 17-(1-oxoheptyl)oxy-, (17.beta.)-
118. Testosterone Enantate, European Pharmacopoeia (ep) Reference Standard
119. Testosterone Enanthate, United States Pharmacopeia (usp) Reference Standard
120. Testosterone Enantate For Peak Identification, European Pharmacopoeia (ep) Reference Standard
121. Testosterone Enantate For System Suitability, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 400.6 g/mol |
|---|---|
| Molecular Formula | C26H40O3 |
| XLogP3 | 6.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 7 |
| Exact Mass | 400.29774513 g/mol |
| Monoisotopic Mass | 400.29774513 g/mol |
| Topological Polar Surface Area | 43.4 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 679 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Testosterone enanthate |
| Drug Label | DELATESTRYL (Testosterone Enanthate Injection, USP) provides testosterone enanthate, a derivative of the primary endogenous androgen testosterone, for intramuscular administration. In their active form, androgens have a 17-beta-hydroxy group. Ester... |
| Active Ingredient | Testosterone enanthate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 200mg/ml |
| Market Status | Prescription |
| Company | Hikma Farmaceutica; Watson Labs; Paddock; Mylan Institutional |
| 2 of 2 | |
|---|---|
| Drug Name | Testosterone enanthate |
| Drug Label | DELATESTRYL (Testosterone Enanthate Injection, USP) provides testosterone enanthate, a derivative of the primary endogenous androgen testosterone, for intramuscular administration. In their active form, androgens have a 17-beta-hydroxy group. Ester... |
| Active Ingredient | Testosterone enanthate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 200mg/ml |
| Market Status | Prescription |
| Company | Hikma Farmaceutica; Watson Labs; Paddock; Mylan Institutional |
Testosterone enanthate in males is indicated as a replacement therapy in conditions associated with a deficiency or absence of endogenous testosterone. Some of the treated conditions are 1) primary hypogonadism, defined as testicular failure due to cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome or orchidectomy; 2) hypogonadotropic hypogonadism due to an idiopathic gonadotropin or luteinizing hormone-releasing hormone deficiency or due to a pituitary-hypothalamic injury from tumors, trauma or radiation, in this case it is important to accompany the treatment with adrenal cortical and thyroid hormone replacement therapy; 3) to stimulate puberty in patients with delayed puberty not secondary to a pathological disorder. If the conditions 1 and 2 occur prior to puberty, the androgen replacement therapy will be needed during adolescent years for the development of secondary sexual characteristics and prolonged androgen treatment might be needed it to maintain sexual characteristics after puberty. In females, testosterone enanthate is indicated to be used secondarily in presence of advanced inoperable metastatic mammary cancer in women who are from one to five years postmenopausal. It has also been used in premenopausal women with breast cancer who have benefited from oophorectomy and are considered to have a hormone-responsive tumor. Testosterone enanthate injections that are currently formulated for subcutaneous use are specifically indicated only for primary hypogonadism and hypogonadotropic hypogonadism. The use of such formulations is limited because the safety and efficacy of these subcutaneous products in adult males with late-onset hypogonadism and males less than 18 years old have not yet been established. Moreover, subcutaneously administered testosterone enanthate is indicated only for the treatment of men with hypogonadal conditions associated with structural or genetic etiologies, considering the medication could cause blood pressure increases that can raise the risk of major adverse cardiovascular events like non-fatal myocardial infarction, non-fatal stroke, and cardiovascular death.
FDA Label
Administration of ester derivatives of testosterone as testosterone enanthate generates an increase in serum testosterone to levels reaching 400% from the baseline within 24 hours of administration. These androgen levels remain elevated for 3-5 days after initial administration. Continuous administration of testosterone enanthate shows a significant suppression of dihydrotestosterone, serum PSA, HDL and FSH, as well as a slight increase in serum estradiol. The levels of dihydrotestosterone and FSH can remain suppressed even 14 days after treatment termination. There are no changes in mood and sexual activity by the presence of testosterone enanthate.
Androgens
Compounds that interact with ANDROGEN RECEPTORS in target tissues to bring about the effects similar to those of TESTOSTERONE. Depending on the target tissues, androgenic effects can be on SEX DIFFERENTIATION; male reproductive organs, SPERMATOGENESIS; secondary male SEX CHARACTERISTICS; LIBIDO; development of muscle mass, strength, and power. (See all compounds classified as Androgens.)
Absorption
The pharmacokinetic profile of testosterone enanthate was studied in a regime of multiple dosing and the testosterone level was reported to present a Cmax above 1200 ng/dl after 24 hours of the last dose. The concentration decreased sequentially until it reached 600 ng/dl after one week. The pharmacokinetic profile of testosterone enanthate presented differences depending on the administered dose in which the tmax was shifted to a range of 36-48 hours. The plasma testosterone level plateaued below the therapeutic range after 3-4 weeks. This reports showed that the different formulation of testosterone enanthate and testosterone cypionate generates a different profile and thus, they are not therapeutically equivalent.
Route of Elimination
About 90% of a dose of testosterone given intramuscularly is excreted in the urine as glucuronic and sulfuric acid conjugates of testosterone and its metabolites; about 6% of a dose is excreted in the feces, mostly in the unconjugated form. The inactivation of testosterone occurs primarily in the liver.
Volume of Distribution
The volume of distribution following intravenous administration of testosterone is of approximately 1 L/kg.
To start its activity, testosterone enanthate has to be processed by enzymes in the bloodstream. These enzymes will catalyze the molecule at the ester location of the moiety. Once processed in this manner, the testosterone enanthate molecule is metabolized to various 17-keto steroids through two different pathways. Subsequently, the major active metabolites are estradiol and DHTd. Testosterone is metabolized to DHT by steroid 5-reductase in skin, liver and urogenital tract. In reproductive tissues DHT is further metabolized to androstanediol.
Testosterone enanthate presents a long half-life in the range of 7-9 days.
The effects of testosterone in humans and other vertebrates occur by way of two main mechanisms: by activation of the androgen receptor (directly or as DHT), and by conversion to estradiol and activation of certain estrogen receptors. Free testosterone (T) is transported into the cytoplasm of target tissue cells, where it can bind to the androgen receptor, or can be reduced to 5α-dihydrotestosterone (DHT) by the cytoplasmic enzyme 5α-reductase. DHT binds to the same androgen receptor even more strongly than T, so that its androgenic potency is about 2.5 times that of T. The T-receptor or DHT-receptor complex undergoes a structural change that allows it to move into the cell nucleus and bind directly to specific nucleotide sequences of the chromosomal DNA. The areas of binding are called hormone response elements (HREs), and influence transcriptional activity of certain genes, producing androgen effects. Such activities are useful as endogenous androgens like testosterone and dihydrotestosterone are responsible for the normal growth and development of the male sex organs and for maintenance of secondary sex characteristics. These effects include the growth and maturation of the prostate, seminal vesicles, penis, and scrotum; the development of male hair distribution, such as facial, pubic, chest, and axillary hair; laryngeal enlargement, vocal cord thickening, and alterations in body musculature and fat distribution. Male hypogonadism, a clinical syndrome resulting from insufficient secretion of testosterone, has two main etiologies. Primary hypogonadism is caused by defects of the gonads, such as Klinefelters syndrome or Leydig cell aplasia, whereas secondary hypogonadism is the failure of the hypothalamus (or pituitary) to produce sufficient gonadotropins (FSH, LH).

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
18
PharmaCompass offers a list of Testosterone Enanthate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Testosterone Enanthate manufacturer or Testosterone Enanthate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Testosterone Enanthate manufacturer or Testosterone Enanthate supplier.
PharmaCompass also assists you with knowing the Testosterone Enanthate API Price utilized in the formulation of products. Testosterone Enanthate API Price is not always fixed or binding as the Testosterone Enanthate Price is obtained through a variety of data sources. The Testosterone Enanthate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Androtardyl manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Androtardyl, including repackagers and relabelers. The FDA regulates Androtardyl manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Androtardyl API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Androtardyl manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Androtardyl supplier is an individual or a company that provides Androtardyl active pharmaceutical ingredient (API) or Androtardyl finished formulations upon request. The Androtardyl suppliers may include Androtardyl API manufacturers, exporters, distributors and traders.
click here to find a list of Androtardyl suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Androtardyl DMF (Drug Master File) is a document detailing the whole manufacturing process of Androtardyl active pharmaceutical ingredient (API) in detail. Different forms of Androtardyl DMFs exist exist since differing nations have different regulations, such as Androtardyl USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Androtardyl DMF submitted to regulatory agencies in the US is known as a USDMF. Androtardyl USDMF includes data on Androtardyl's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Androtardyl USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Androtardyl suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Androtardyl Drug Master File in Japan (Androtardyl JDMF) empowers Androtardyl API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Androtardyl JDMF during the approval evaluation for pharmaceutical products. At the time of Androtardyl JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Androtardyl suppliers with JDMF on PharmaCompass.
A Androtardyl CEP of the European Pharmacopoeia monograph is often referred to as a Androtardyl Certificate of Suitability (COS). The purpose of a Androtardyl CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Androtardyl EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Androtardyl to their clients by showing that a Androtardyl CEP has been issued for it. The manufacturer submits a Androtardyl CEP (COS) as part of the market authorization procedure, and it takes on the role of a Androtardyl CEP holder for the record. Additionally, the data presented in the Androtardyl CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Androtardyl DMF.
A Androtardyl CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Androtardyl CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Androtardyl suppliers with CEP (COS) on PharmaCompass.
A Androtardyl written confirmation (Androtardyl WC) is an official document issued by a regulatory agency to a Androtardyl manufacturer, verifying that the manufacturing facility of a Androtardyl active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Androtardyl APIs or Androtardyl finished pharmaceutical products to another nation, regulatory agencies frequently require a Androtardyl WC (written confirmation) as part of the regulatory process.
click here to find a list of Androtardyl suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Androtardyl as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Androtardyl API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Androtardyl as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Androtardyl and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Androtardyl NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Androtardyl suppliers with NDC on PharmaCompass.
Androtardyl Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Androtardyl GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Androtardyl GMP manufacturer or Androtardyl GMP API supplier for your needs.
A Androtardyl CoA (Certificate of Analysis) is a formal document that attests to Androtardyl's compliance with Androtardyl specifications and serves as a tool for batch-level quality control.
Androtardyl CoA mostly includes findings from lab analyses of a specific batch. For each Androtardyl CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Androtardyl may be tested according to a variety of international standards, such as European Pharmacopoeia (Androtardyl EP), Androtardyl JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Androtardyl USP).

