
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Listed Suppliers
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
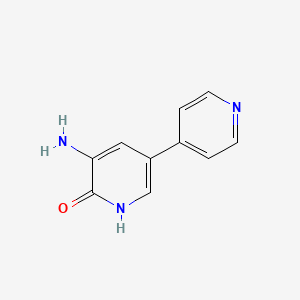

1. 5-amino-(3,4'-bipyridine)-6(1h)-one
2. Amrinon
3. Cordemcura
4. Inocor
5. Win 40680
6. Win-40680
7. Win40680
8. Wincoram
1. 60719-84-8
2. Inamrinone
3. Wincoram
4. Inocor
5. Cordemcura
6. Cartonic
7. Amcoral
8. Amrinonum [inn-latin]
9. Vesistol
10. 5-amino-[3,4'-bipyridin]-6(1h)-one
11. Amrinona [inn-spanish]
12. 3-amino-5-(4-pyridinyl)-2(1h)-pyridinone
13. Win-40680
14. Win 40680
15. 3-amino-5-pyridin-4-yl-1h-pyridin-2-one
16. 5-amino-(3,4'-bipyridin)-6(1h)-one
17. Awd 08-250
18. [3,4'-bipyridin]-6(1h)-one, 5-amino-
19. Amrinone [inn]
20. 5-amino-3,4'-bipyridin-6(1h)-one
21. Amrinone Lactate
22. Mls000069829
23. 5-amino(3,4'-bipyridin)-6(1h)-one
24. Nsc-759805
25. (3,4'-bipyridin)-6(1h)-one, 5-amino-
26. Smr000058850
27. Chembl12856
28. Jut23379tn
29. C01ce01
30. 3-amino-5-pyridin-4-ylpyridin-2-ol
31. 3-amino-5-(pyridin-4-yl)pyridin-2-ol
32. 5-amino[3,4'-bipyridin]-6(1h)-one
33. Ncgc00164379-01
34. Amrinona
35. Amrinonum
36. Cas-60719-84-8
37. Dsstox_cid_2603
38. Dsstox_rid_76655
39. Dsstox_gsid_22603
40. Inamrinone (usp)
41. Amcoral (tn)
42. Ccris 3794
43. 3-amino-5-(pyridin-4-yl)pyridin-2(1h)-one
44. Amrinone (jan/inn)
45. Inamrinone [usan:usp]
46. Einecs 262-390-0
47. Brn 0744819
48. Unii-jut23379tn
49. Prestwick_44
50. Amrinone,(s)
51. Mfcd00083228
52. Spectrum_001350
53. Amrinone [jan]
54. Amrinone [mi]
55. Amrinone [vandf]
56. Opera_id_1054
57. Prestwick0_000800
58. Prestwick1_000800
59. Prestwick2_000800
60. Prestwick3_000800
61. Spectrum2_001980
62. Spectrum3_000956
63. Spectrum4_001069
64. Spectrum5_000999
65. Inamrinone [usan]
66. Amrinone [mart.]
67. Inamrinone [vandf]
68. Amrinone [usp-rs]
69. Amrinone [who-dd]
70. Cid_3698
71. Schembl44012
72. Bspbio_000940
73. Kbiogr_001398
74. Kbioss_001830
75. 5-25-15-00181 (beilstein Handbook Reference)
76. Mls001074083
77. Divk1c_000136
78. Spectrum1503084
79. Spbio_002139
80. Spbio_002879
81. Bpbio1_001034
82. Chebi:2686
83. Gtpl7202
84. Dtxsid9022603
85. Schembl13457017
86. Bdbm34651
87. Hms500g18
88. Kbio1_000136
89. Kbio2_001830
90. Kbio2_004398
91. Kbio2_006966
92. Kbio3_002052
93. Inamrinone [usp Impurity]
94. Ninds_000136
95. Hms1570o22
96. Hms2097o22
97. Hms2234f12
98. Hms3264e16
99. Hms3369j10
100. Hms3714o22
101. Inamrinone [usp Monograph]
102. Pharmakon1600-01503084
103. Bcp12764
104. Hy-b1294
105. Zinc8673078
106. Tox21_112110
107. Tox21_301847
108. Ccg-39487
109. Nsc759805
110. Stk590307
111. Akos005512516
112. Tox21_112110_1
113. Db01427
114. Ks-5178
115. Nsc 759805
116. Cid 5281003
117. Idi1_000136
118. Ncgc00016896-01
119. Ncgc00016896-02
120. Ncgc00016896-03
121. Ncgc00016896-04
122. Ncgc00016896-06
123. Ncgc00095984-01
124. Ncgc00255137-01
125. Ac-12186
126. Ac-33137
127. Ba166404
128. 5-amino(3,4'-bipyridine)-6-(1h)-one
129. 5-amino-(3,4?-bipyridin)-6(1h)-one
130. 3-amino-5-(4-pyridinyl)-2(1h)pyridinone
131. 3-azanyl-5-pyridin-4-yl-1h-pyridin-2-one
132. Cs-0013064
133. Ft-0601595
134. 5-amino-3,4'-bipyridyl-6(1h)-one
135. C75837
136. D00231
137. Ab00052312-04
138. Ab00052312_05
139. 3-amino-5-(4-pyridinyl)-1,2-dihydro-2-pyridone
140. 719a848
141. A832852
142. Q422724
143. Sr-00000002445
144. Sr-00000002445-2
145. W-105234
146. 3-amino-5-(pyridin-4-yl)-1,2-dihydropyridin-2-one
147. Brd-k45924332-001-04-4
148. Brd-k45924332-001-07-7
149. Amrinone, United States Pharmacopeia (usp) Reference Standard
150. 216151-27-8
| Molecular Weight | 187.20 g/mol |
|---|---|
| Molecular Formula | C10H9N3O |
| XLogP3 | -0.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 187.074561919 g/mol |
| Monoisotopic Mass | 187.074561919 g/mol |
| Topological Polar Surface Area | 68 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 301 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used in the treatment of congestive heart failure.
Amrinone is a positive inotropic cardiotonic with vasodilator properties, phosphodiesterase inhibitory activity, and the ability to stimulate calcium ion influx into the cardiac cell.
Calcium Channel Blockers
A class of drugs that act by selective inhibition of calcium influx through cellular membranes. (See all compounds classified as Calcium Channel Blockers.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Cardiotonic Agents
Agents that have a strengthening effect on the heart or that can increase cardiac output. They may be CARDIAC GLYCOSIDES; SYMPATHOMIMETICS; or other drugs. They are used after MYOCARDIAL INFARCT; CARDIAC SURGICAL PROCEDURES; in SHOCK; or in congestive heart failure (HEART FAILURE). (See all compounds classified as Cardiotonic Agents.)
Phosphodiesterase 3 Inhibitors
Compounds that specifically inhibit PHOSPHODIESTERASE 3. (See all compounds classified as Phosphodiesterase 3 Inhibitors.)
C - Cardiovascular system
C01 - Cardiac therapy
C01C - Cardiac stimulants excl. cardiac glycosides
C01CE - Phosphodiesterase inhibitors
C01CE01 - Amrinone
Route of Elimination
The primary route of excretion in man is via the urine as both inamrinone and several metabolites (N-glycolyl, N-acetate, O-glucuronide and N-glucuronide).
Volume of Distribution
1.2 L/kg [normal volunteers]
Hepatic.
5 to 8 hours
Amrinone is a phosphodiesterase inhibitor (PDE3), resulting in increased cAMP and cGMP which leads to an increase in the calcium influx like that caused by beta-agonists resulting in increased inotropic effect.
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
68
PharmaCompass offers a list of Amrinone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Amrinone manufacturer or Amrinone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Amrinone manufacturer or Amrinone supplier.
PharmaCompass also assists you with knowing the Amrinone API Price utilized in the formulation of products. Amrinone API Price is not always fixed or binding as the Amrinone Price is obtained through a variety of data sources. The Amrinone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Amrinone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Amrinone, including repackagers and relabelers. The FDA regulates Amrinone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Amrinone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Amrinone supplier is an individual or a company that provides Amrinone active pharmaceutical ingredient (API) or Amrinone finished formulations upon request. The Amrinone suppliers may include Amrinone API manufacturers, exporters, distributors and traders.
click here to find a list of Amrinone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Amrinone DMF (Drug Master File) is a document detailing the whole manufacturing process of Amrinone active pharmaceutical ingredient (API) in detail. Different forms of Amrinone DMFs exist exist since differing nations have different regulations, such as Amrinone USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Amrinone DMF submitted to regulatory agencies in the US is known as a USDMF. Amrinone USDMF includes data on Amrinone's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Amrinone USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Amrinone suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Amrinone as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Amrinone API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Amrinone as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Amrinone and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Amrinone NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Amrinone suppliers with NDC on PharmaCompass.
Amrinone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Amrinone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Amrinone GMP manufacturer or Amrinone GMP API supplier for your needs.
A Amrinone CoA (Certificate of Analysis) is a formal document that attests to Amrinone's compliance with Amrinone specifications and serves as a tool for batch-level quality control.
Amrinone CoA mostly includes findings from lab analyses of a specific batch. For each Amrinone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Amrinone may be tested according to a variety of international standards, such as European Pharmacopoeia (Amrinone EP), Amrinone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Amrinone USP).

