


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
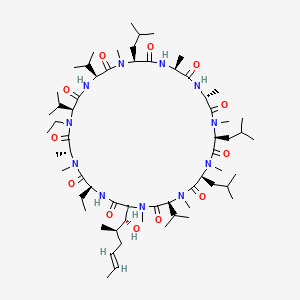

1. Debio-025
2. Meala(3)etval(4)-cyclosporin
3. Unil 025
4. Unil-025
5. Unil025
1. Debio-025
2. 254435-95-5
3. Debio 025
4. Unil 025
5. Unil-025
6. Deb-025
7. Deb025
8. (d-meala)3-(etval)4-csa
9. Vbp9099aa6
10. Chembl1651956
11. Deb 025
12. Alisporivir (usan)
13. Alisporivir [usan]
14. (3s,6s,9s,12r,15s,18s,21s,24s,27r,30s,33s)-25,30-diethyl-33-((1r,2r,e)-1-hydroxy-2-methylhex-4-en-1-yl)-6,9,18-triisobutyl-3,21,24-triisopropyl-1,4,7,10,12,15,19,27,28-nonamethyl-1,4,7,10,13,16,19,22,25,28,31-undecaazacyclotritriacontan-2,5,8,11,14,17,20,23,26,29,32-undecaone
15. Cyclo(l-alanyl-d-alanyl-n-methyl-l-leucyl-n-methyl-l-leucyl-n-methyl-l-valyl-(2s,3r,4r,6e)-3-hydroxy-4-methyl-2-(methylamino)-6-octenoyl-(2s)-2-aminobutanoyl-n-methyl-d-alanyl-n-ethyl-l-valyl-l-valyl-n-methyl-l-leucyl)
16. Alisporivir [inn]
17. Alisporivir [usan:inn]
18. Unii-vbp9099aa6
19. Unil025
20. Meala(3)etval(4)-cyclosporin
21. Alisporivir [who-dd]
22. Debio-025;deb-025
23. Schembl6850787
24. Chebi:149837
25. Dtxsid401027752
26. Bdbm50339127
27. De-025
28. Cs-7501
29. Db12139
30. Hy-12559
31. D10087
32. Q4727236
33. (3s,6s,9s,12r,15s,18s,21s,24s,27r,30s,33s)-25,30-diethyl-33-((1r,2r)-1-hydroxy-2-methylhex-4-enyl)-6,9,18-triisobutyl-3,21,24-triisopropyl-1,4,7,10,12,15,19,27,28-nonamethyl-1,4,7,10,13,16,19,22,25,28,31-undecaazacyclotritriacontan-2,5,8,11,14,17,20,23,26,29,32-undecaone
34. (3s,6s,9s,12r,15s,18s,21s,24s,27r,30s,33s)-25,30-diethyl-33-((1r,2r,e)-1-hydroxy-2-methylhex-4-enyl)-6,9,18-triisobutyl-3,21,24-triisopropyl-1,4,7,10,12,15,19,27,28-nonamethyl-1,4,7,10,13,16,19,22,25,28,31-undecaazacyclotritriacontan-2,5,8,11,14,17,20,23,26,29,32-undecaone
35. (3s,6s,9s,12r,15s,18s,21s,24s,27r,30s,33s)-25,30-diethyl-33-[(1r,2r,4e)-1-hydroxy-2-methylhex-4-en-1-yl]-1,4,7,10,12,15,19,27,28-nonamethyl-6,9,18-tris(2-methylpropyl)-3,21,24-tris(propan-2-yl)-1,4,7,10,13,16,19,22,25,28,31-undecaazacyclotritriacontan-2,5,8,11,14,17,20,23,26,29,32-undecone
36. (3s,6s,9s,12r,15s,18s,21s,24s,27r,30s,33s)-25,30-diethyl-33-[(e,1r,2r)-1-hydroxy-2-methylhex-4-enyl]-1,4,7,10,12,15,19,27,28-nonamethyl-6,9,18-tris(2-methylpropyl)-3,21,24-tri(propan-2-yl)-1,4,7,10,13,16,19,22,25,28,31-undecazacyclotritriacontane-2,5,8,11,14,17,20,23,26,29,32-undecone
| Molecular Weight | 1216.6 g/mol |
|---|---|
| Molecular Formula | C63H113N11O12 |
| XLogP3 | 7.9 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 15 |
| Exact Mass | 1215.85701808 g/mol |
| Monoisotopic Mass | 1215.85701808 g/mol |
| Topological Polar Surface Area | 279 Ų |
| Heavy Atom Count | 86 |
| Formal Charge | 0 |
| Complexity | 2360 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 13 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of chronic hepatitis C


