
Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Canada
0
Australia
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
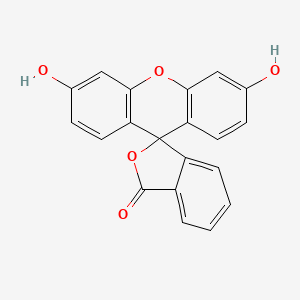

1. C.i. 45350
2. Colircusi Fluoresceina
3. D And C Yellow No. 7
4. D And C Yellow No. 8
5. Diofluor
6. Dipotassium Salt, Fluorescein
7. Disodium Fluorescein
8. Disodium Salt, Fluorescein
9. Fluor I Strip A.t.
10. Fluor-i-strip A.t.
11. Fluorescine Sodique Faure
12. Fluorescein Dipotassium Salt
13. Fluorescein Disodium Salt
14. Fluorescein Monosodium Salt
15. Fluorescein Sodium, Minims
16. Fluorescein, Disodium
17. Fluorescein, Sodium
18. Fluoresceina, Colircusi
19. Fluoresceine, Minims
20. Fluorescite
21. Fluorets
22. Ful Glo
23. Ful-glo
24. Funduscein
25. Minims Fluorescein Sodium
26. Minims Fluoresceine
27. Minims Stains
28. Monosodium Salt, Fluorescein
29. Optifluor Diba
30. Sodium Fluorescein
31. Sodium, Fluorescein
32. Uranine
1. 2321-07-5
2. Solvent Yellow 94
3. Resorcinolphthalein
4. Yellow Fluorescein
5. Fluoresceine
6. 3',6'-dihydroxy-3h-spiro[isobenzofuran-1,9'-xanthen]-3-one
7. 3,6-fluorandiol
8. D&c Yellow No. 7
9. Japan Yellow 201
10. D And C Yellow No. 7
11. C.i. Solvent Yellow 94
12. 3',6'-dihydroxyfluoran
13. Fluorescein Acid
14. Fluoreszein
15. Soap Yellow F
16. Hidacid Fluorescein
17. Resorcinol Phthalein
18. D & C Yellow No. 7
19. D+c Yellow No. 7
20. 9-(o-carboxyphenyl)-6-hydroxy-3-isoxanthenone
21. Fluorescein (free Acid)
22. 11712 Yellow
23. Chebi:31624
24. 9-(o-carboxyphenyl)-6-hydroxy-3h-xanthen-3-one
25. Japan Yellow No. 201
26. Zlut Kysela 73
27. 3',6'-dihydroxyspiro[2-benzofuran-3,9'-xanthene]-1-one
28. Fluoran, 3',6'-dihydroxy-
29. Nsc 667256
30. 3',6'-fluorandiol
31. C.i. 45350:1
32. Mfcd00005050
33. Tpy09g7xir
34. Spiro[isobenzofuran-1(3h),9'-[9h]xanthen]-3-one, 3',6'-dihydroxy-
35. Chembl1057
36. Ci 45350:1
37. 3h-xanthen-3-one, 9-(o-carboxyphenyl)-6-hydroxy-
38. Nsc667256
39. Benzoic Acid, O-(6-hydroxy-3-oxo-3h-xanthen-9-yl)-
40. Nsc-667256
41. Nsc-759114
42. Benzoic Acid, 2-(6-hydroxy-3-oxo-3h-xanthen-9-yl)-
43. Ncgc00161643-03
44. 3',6'-dihydroxyspiro(isobenzofuran-1(3h),9'(9h)-xanthen)-3-one
45. 3',6'-dihydroxyspiro(isobenzofuran-1(3h),9'-(9h)xanthen)-3-one
46. Spiro(isobenzofuran-1(3h),9'-(9h)xanthen)-3-one, 3',6'-dihydroxy-
47. 3,6-dihydroxyspiro(xanthene-9,3'-phthalide)
48. Dsstox_cid_18887
49. Dsstox_rid_79416
50. Dsstox_gsid_38887
51. 3',6'-dihydroxy-3h-spiro[2-benzofuran-1,9'-xanthen]-3-one
52. Fluorescein Red
53. Cas-2321-07-5
54. 3,6-dihydroxyspiro[xanthene-9,3'-phthalide]
55. Zlut Kysela 73 [czech]
56. Fluorecein
57. Diofluor
58. Ccris 7076
59. Hsdb 2128
60. Fluorescite (tn)
61. Diresorcinolphthalein
62. Einecs 219-031-8
63. Brn 0094324
64. Fluorescite (salt/mix)
65. 3',6' Dihydroxyfluoran
66. Fluorescein (jan/usp)
67. Fluorescein [ii]
68. Fluorescein [mi]
69. Unii-tpy09g7xir
70. Fluorescein [jan]
71. Fluorescein [hsdb]
72. Epitope Id:137337
73. Upcmld-dp087
74. Yellow 7 [inci]
75. Fluorescein [vandf]
76. Fluorescein [mart.]
77. Schembl16533
78. Fluorescein [usp-rs]
79. Fluorescein [who-dd]
80. Fluorescein [usp:ban:jan]
81. Ki201 [inci]
82. Dtxsid0038887
83. Upcmld-dp087:001
84. Acid Yellow 73 [inci]
85. Gnbhrkfjiuuoqi-uhfffaoysa-
86. Fluorescein (solvent Yellow 94)
87. C.i. 45350 (salt/mix)
88. Fluorescein [ep Monograph]
89. Hms2093f21
90. Hms3744c17
91. Moli001003
92. Pharmakon1600-01505396
93. Fluorescein [usp Monograph]
94. Amy22388
95. Hy-d0251
96. Zinc3860453
97. Tox21_113500
98. Tox21_303508
99. Bdbm50237588
100. C.i. Acid Yellow 73 (salt/mix)
101. Nsc759114
102. S5488
103. Spiro(isobenzofuran-1(3h),9'-(9h)xanthen)-3-one,3'6'-dihydroxy-
104. Akos015903296
105. Ccg-213417
106. Cs-7537
107. Db00693
108. C.i. 45350a
109. Fluorescein, For Fluorescence, Free Acid
110. Ncgc00161643-01
111. Ncgc00161643-02
112. Ncgc00257491-01
113. D & C Yellow No. 7 K7133
114. Sy012645
115. Sbi-0206827.p001
116. F0095
117. Fluorescein (free Acid), Dye Content 95 %
118. Ft-0668579
119. D01261
120. D70685
121. Ec 219-031-8
122. Ab00643381_02
123. 5-19-06-00456 (beilstein Handbook Reference)
124. Q410922
125. Sr-05000001696
126. Sr-05000001696-1
127. W-107417
128. Brd-k21913543-001-01-6
129. 3',6'-dihydroxyspiro[isobenzofuran-3,9'-xanthene]-1-one
130. E89fa0b4-4844-46a2-af9b-29eca50e5599
131. Fluorescein, European Pharmacopoeia (ep) Reference Standard
132. 3',6'-dihydroxy-3h-spiro[isobenzo[b]furan-1,9'-xanthen]-3-one
133. Fluorescein, United States Pharmacopeia (usp) Reference Standard
134. Fluorescein Low Range Dna Standard, Fluorescein-labeled Marker For Dna Electrophoresis
135. Spiro[isobenzofuran-1(3h),9'-[9h]xanthene]-ar-carboxylicacid, 3',6'-dihydroxy-3-oxo-
| Molecular Weight | 332.3 g/mol |
|---|---|
| Molecular Formula | C20H12O5 |
| XLogP3 | 3.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 0 |
| Exact Mass | 332.06847348 g/mol |
| Monoisotopic Mass | 332.06847348 g/mol |
| Topological Polar Surface Area | 76 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 522 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Fluorescite |
| PubMed Health | Fluorescein (Injection) |
| Drug Classes | Disclosing Agent |
| Drug Label | FLUORESCITE (fluorescein injection, USP) 10% contains fluorescein sodium (equivalent to fluorescein 10% w/v). It is a sterile solution for use intravenously as a diagnostic aid. Its chemical name is spiro[isobenzofuran-1(3H), 9'-[9H]xanthene]-3-one.. |
| Active Ingredient | Fluorescein sodium |
| Dosage Form | Injectable |
| Route | Intravenous |
| Strength | eq 500mg base/5ml (eq 100mg base/ml) |
| Market Status | Prescription |
| Company | Alcon Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Fluorescite |
| PubMed Health | Fluorescein (Injection) |
| Drug Classes | Disclosing Agent |
| Drug Label | FLUORESCITE (fluorescein injection, USP) 10% contains fluorescein sodium (equivalent to fluorescein 10% w/v). It is a sterile solution for use intravenously as a diagnostic aid. Its chemical name is spiro[isobenzofuran-1(3H), 9'-[9H]xanthene]-3-one.. |
| Active Ingredient | Fluorescein sodium |
| Dosage Form | Injectable |
| Route | Intravenous |
| Strength | eq 500mg base/5ml (eq 100mg base/ml) |
| Market Status | Prescription |
| Company | Alcon Pharms |
Contrast Medium
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
VET: Deep corneal ulcers, descemetocele, and iris prolapse are seen with some frequency in dogs, cats, and horses. ... Important diagnostic aids are the Schirmer tear test to measure aqueous tear production and topical fluorescein to examine the corneal ulcer. ...
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 1416
Fluorescein is now sometimes used for determination of circulation time, adequacy of blood supply, and viability of tissue.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1004
In determination of circulation time, ... by rapid iv ... appearance of fluorescence in lips, eyes, or intact skin or in wheals (histamine or scratch) ... is taken as end point.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1004
Measurement of arm-to-retina circulation time is employed for diagnosis of carotid artery occlusion.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 985
Adverse effects following topical administration to the eye may include irritation and rash. Fluorescein may cause yellow discoloration of skin or eyes. urine may attain a bright yellow color. Adverse effects following intravenous administration include nausea, vomiting, headache, dizziness, fainting, and low blood pressure.
United States Pharmacopeial Convention, Inc (USP); MSDS Database Online; Material Safety Data Sheet: Fluorescein; Catalog Number: 1277004; (Revision Date: January 31, 2005)
For diagnostic imaging. Primarily indicated in diagnostic fluorescein angiography or angioscopy of the fundus and of the iris vasculature.
Contrast Media
Substances used to allow enhanced visualization of tissues. (See all compounds classified as Contrast Media.)
Fluorescent Dyes
Chemicals that emit light after excitation by light. The wave length of the emitted light is usually longer than that of the incident light. Fluorochromes are substances that cause fluorescence in other substances, i.e., dyes used to mark or label other compounds with fluorescent tags. (See all compounds classified as Fluorescent Dyes.)
S01JA01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
S - Sensory organs
S01 - Ophthalmologicals
S01J - Diagnostic agents
S01JA - Colouring agents
S01JA01 - Fluorescein
Absorption
Rapidly distributed
Route of Elimination
Fluorescein and its metabolites are mainly eliminated via renal excretion.
Volume of Distribution
0.5 L/kg
Clearance
renal cl=1.75 mL/min/kg [After IV administration]
hepatic cl=1.50 mL/min/kg [After IV administration]
Fluorescence of skin persists for several hr, and dye appears in urine for as long as 30 hr.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1004
Within 7 to 14 seconds after IV administration into antecubital vein, fluorescein usually appears in the central artery of the eye. Within a few minutes of IV administration of fluorescein sodium, a yellowish discoloration of the skin occurs, which begins to fade after 6 to 12 hours of dosing. Various estimates of volume of distribution indicate that fluorescein distributes well into interstitial space (0.5 L/ kg).
US Natl Inst Health; DailyMed. Current Medication Information for Fluorescite (fluorescein sodium) injection, solution (2009). Available from, as of October 19, 2009: https://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=Fluorescein+sodium
Fluorescein and its metabolites are mainly eliminated via renal excretion. After IV administration, the urine remains slightly fluorescent for 24 to 36 hours. A renal clearance of 1.75 mL/min/kg and a hepatic clearance (due to conjugation) of 1.50 mL/min/kg have been estimated. The systemic clearance of fluorescein was essentially complete by 48 to 72 hours after administration of 500 mg fluorescein.
US Natl Inst Health; DailyMed. Current Medication Information for Fluorescite (fluorescein sodium) injection, solution (2009). Available from, as of October 19, 2009: https://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=Fluorescein+sodium
Fluorescein sodium has been demonstrated to be excreted in human milk.
US Natl Inst Health; DailyMed. Current Medication Information for Fluorescite (fluorescein sodium) injection, solution (2009). Available from, as of October 19, 2009: https://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=Fluorescein+sodium
The permeability of the blood-retinal and blood-aqueous barriers to fluorescein and the rate of aqueous flow can be estimated by measurements of fluorescein in the vitreous, aqueous, and plasma after systemic administration. Fluorescein is commonly measured by fluorescence, but fluorescein glucuronide, a metabolite of fluorescein, also fluoresces. To assess the influence of fluorescein glucuronide on the quantitation of fluorescein by fluorescence, we studied the pharmacokinetics of fluorescein and fluorescein glucuronide for 38 hr in the plasma of five normal subjects given 14 mg/kg of sodium fluorescein intravenously. The plasma and the plasma ultrafiltrate were measured by fluorescence and by high performance liquid chromatography. In our fluorophotometer, fluorescein glucuronide was 0.124 times as fluorescent as fluorescein. Fluorescein was rapidly converted to fluorescein glucuronide, and within 10 min the concentration of unbound fluorescein glucuronide exceeded that of unbound fluorescein. The terminal half-lives of fluorescein and fluorescein glucuronide in the plasma ultrafiltrate were 23.5 and 264 min, respectively, so that fluorescein glucuronide contributed almost all of the plasma fluorescence after 4-5 hr. Because fluorescein glucuronide was less bound in the plasma than fluorescein, the ratio of the fluorescence of the plasma ultrafiltrate to that of the plasma increased with time. The greatest proportion of the total fluorescein available to penetrate into the ocular compartments occurred shortly after injection. ...
PMID:3721789 Blair NP et al; Invest Ophthalmol Vis Sci 27 (7): 1107-14 (1986).
The permeability of the blood-retinal and blood-aqueous barriers to fluorescein and the rate of aqueous flow can be estimated by measurements of fluorescein in the vitreous, aqueous, and plasma after systemic administration. Fluorescein is commonly measured by fluorescence, but fluorescein glucuronide, a metabolite of fluorescein, also fluoresces. To assess the influence of fluorescein glucuronide on the quantitation of fluorescein by fluorescence, we studied the pharmacokinetics of fluorescein and fluorescein glucuronide for 38 hr in the plasma of five normal subjects given 14 mg/kg of sodium fluorescein intravenously. The plasma and the plasma ultrafiltrate were measured by fluorescence and by high performance liquid chromatography. In our fluorophotometer, fluorescein glucuronide was 0.124 times as fluorescent as fluorescein. Fluorescein was rapidly converted to fluorescein glucuronide, and within 10 min the concentration of unbound fluorescein glucuronide exceeded that of unbound fluorescein. The terminal half-lives of fluorescein and fluorescein glucuronide in the plasma ultrafiltrate were 23.5 and 264 min, respectively, so that fluorescein glucuronide contributed almost all of the plasma fluorescence after 4-5 hr. Because fluorescein glucuronide was less bound in the plasma than fluorescein, the ratio of the fluorescence of the plasma ultrafiltrate to that of the plasma increased with time. The greatest proportion of the total fluorescein available to penetrate into the ocular compartments occurred shortly after injection. ...
PMID:3721789 Blair NP et al; Invest Ophthalmol Vis Sci 27 (7): 1107-14 (1986).
Fluorescein undergoes rapid metabolism to fluorescein monoglucuronide. After IV administration of fluorescein sodium (14 mg/kg) to 7 healthy subjects, approximately 80% of fluorescein in plasma was converted to glucuronide conjugate after a period of 1 hour post dose, indicating relatively rapid conjugation.
US Natl Inst Health; DailyMed. Current Medication Information for Fluorescite (fluorescein sodium) injection, solution (2009). Available from, as of October 19, 2009: https://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=Fluorescein+sodium
Fluorescein is a known human metabolite of zinc15020070.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
... The pharmacokinetics of fluorescein and fluorescein glucuronide /were studied/ for 38 hr in the plasma of five normal subjects given 14 mg/kg of sodium fluorescein intravenously. ... The terminal half-lives of fluorescein and fluorescein glucuronide in the plasma ultrafiltrate were 23.5 and 264 min, respectively, ... .
PMID:3721789 Blair NP et al; Invest Ophthalmol Vis Sci 27 (7): 1107-14 (1986).
Fluorescein sodium is used extensively as a diagnostic tool in the field of ophthalmology. Fluorescein is a fluorescent compound or fluorophore having a maximum absorbance of 494 m and an emission maximum of 521 nm. The yellowish-green fluorescence of the compound can be used to demarcate the vascular area under observation, distinguishing it from adjacent areas. It is applied topically in the form of a drop or it can be injected intravenously to produce a fluorescein angiogram. Topical fluorescein is a useful tool in the diagnosis of corneal abrasions, corneal ulcers, herpetic corneal infections, and dry eye. Fluorescein angiography is used to diagnose and categorize macular degeneration, diabetic retinopathy, inflammatory intraocular conditions, and intraocular tumors.
Fluorescein sodium responds to electromagnetic radiation and light between the wavelengths of 465-490 nm and fluoresces, i.e., emits light at wavelengths of 520-530 nm. Thus, the hydrocarbon is excited by blue light and emits light that appears yellowish-green. Following intravenous injection of fluorescein sodium in an aqueous solution, the unbound fraction of the fluorescein can be excited with a blue light flash from a fundus camera as it circulates through the ocular vasculature, and the yellowish green fluorescence of the dye is captured by the camera. In the fundus, the fluorescence of the dye demarcates the retinal and/or choroidal vasculature under observation, distinguishing it from adjacent areas/structures. /Fluorescein sodium/
US Natl Inst Health; DailyMed. Current Medication Information for Fluorescite (fluorescein sodium) injection, solution (2009). Available from, as of October 19, 2009: https://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=Fluorescein+sodium

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
85
PharmaCompass offers a list of Fluorescein API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Fluorescein manufacturer or Fluorescein supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Fluorescein manufacturer or Fluorescein supplier.
PharmaCompass also assists you with knowing the Fluorescein API Price utilized in the formulation of products. Fluorescein API Price is not always fixed or binding as the Fluorescein Price is obtained through a variety of data sources. The Fluorescein Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A AK-FLUOR 25% manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of AK-FLUOR 25%, including repackagers and relabelers. The FDA regulates AK-FLUOR 25% manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. AK-FLUOR 25% API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of AK-FLUOR 25% manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A AK-FLUOR 25% supplier is an individual or a company that provides AK-FLUOR 25% active pharmaceutical ingredient (API) or AK-FLUOR 25% finished formulations upon request. The AK-FLUOR 25% suppliers may include AK-FLUOR 25% API manufacturers, exporters, distributors and traders.
click here to find a list of AK-FLUOR 25% suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A AK-FLUOR 25% DMF (Drug Master File) is a document detailing the whole manufacturing process of AK-FLUOR 25% active pharmaceutical ingredient (API) in detail. Different forms of AK-FLUOR 25% DMFs exist exist since differing nations have different regulations, such as AK-FLUOR 25% USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A AK-FLUOR 25% DMF submitted to regulatory agencies in the US is known as a USDMF. AK-FLUOR 25% USDMF includes data on AK-FLUOR 25%'s chemical properties, information on the facilities and procedures used, and details about packaging and storage. The AK-FLUOR 25% USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of AK-FLUOR 25% suppliers with USDMF on PharmaCompass.
A AK-FLUOR 25% CEP of the European Pharmacopoeia monograph is often referred to as a AK-FLUOR 25% Certificate of Suitability (COS). The purpose of a AK-FLUOR 25% CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of AK-FLUOR 25% EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of AK-FLUOR 25% to their clients by showing that a AK-FLUOR 25% CEP has been issued for it. The manufacturer submits a AK-FLUOR 25% CEP (COS) as part of the market authorization procedure, and it takes on the role of a AK-FLUOR 25% CEP holder for the record. Additionally, the data presented in the AK-FLUOR 25% CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the AK-FLUOR 25% DMF.
A AK-FLUOR 25% CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. AK-FLUOR 25% CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of AK-FLUOR 25% suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing AK-FLUOR 25% as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for AK-FLUOR 25% API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture AK-FLUOR 25% as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain AK-FLUOR 25% and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a AK-FLUOR 25% NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of AK-FLUOR 25% suppliers with NDC on PharmaCompass.
AK-FLUOR 25% Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of AK-FLUOR 25% GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right AK-FLUOR 25% GMP manufacturer or AK-FLUOR 25% GMP API supplier for your needs.
A AK-FLUOR 25% CoA (Certificate of Analysis) is a formal document that attests to AK-FLUOR 25%'s compliance with AK-FLUOR 25% specifications and serves as a tool for batch-level quality control.
AK-FLUOR 25% CoA mostly includes findings from lab analyses of a specific batch. For each AK-FLUOR 25% CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
AK-FLUOR 25% may be tested according to a variety of international standards, such as European Pharmacopoeia (AK-FLUOR 25% EP), AK-FLUOR 25% JP (Japanese Pharmacopeia) and the US Pharmacopoeia (AK-FLUOR 25% USP).

