


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
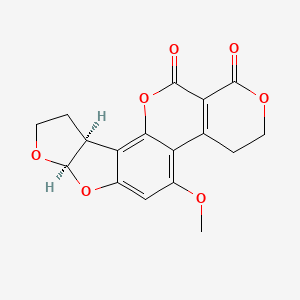

1. 7241-98-7
2. Dihydroaflatoxin G1
3. 2ms0d8wa29
4. (7ar-cis)-3,4,7a,9,10,10a-hexahydro-5-methoxy-1h,12h-furo[3',2':4,5]furo[2,3-h]pyrano[3,4-c][1]benzopyran-1,12-dione
5. Aflatoxin G2 0.5 Microg/ml In Acetonitrile
6. 1h,12h-furo[3',2':4,5]furo[2,3-h]pyrano[3,4-c][1]benzopyran-1,12-dione, 3,4,7a,9,10,10a-hexahydro-5-methoxy-, (7ar,10as)-
7. Aflatoxing2
8. Unii-2ms0d8wa29
9. Ccris 4936
10. Hsdb 3456
11. 1h,12h-furo(3',2':4,5)furo(2,3-h)pyrano(3,4-c)(1)benzopyran-1,12-dione, 3,4,7a,9,10,10a-hexahydro-5-methoxy-, (7ar,10as)-
12. Einecs 230-643-4
13. Aflatoxin G2 [mi]
14. Aflatoxin G2 [hsdb]
15. Aflatoxin G2 In Acetonitrile
16. Chebi:80705
17. Aflatoxin G2, Reference Material
18. Dtxsid80891796
19. Ex-a4123
20. Hy-n6698
21. Zinc2029390
22. Mfcd00078141
23. 1h,12h-furo(3',2':4,5)furo(2,3-h)pyrano(3,4-c)(1)benzopyran-1,12-dione,3,4,7aalpha,9,10,10aalpha-hexahydro-5-methoxy-
24. 1h,12h-furo[3',2':4,5]furo[2,3-h]pyrano[3,4-c][1]benzopyran-1,12-dione, 3,4,7a,9,10,10a-hexahydro-5-
25. 3,4,7aalpha,9,10,10aalpha-hexahydro-5-methoxy-1h,12h-furo(3',2':4,5)furo(2,3-h)pyrano(3,4-c)(1)-benzopyran-1,12-dione
26. Cs-0099736
27. C16754
28. Q26841280
29. Aflatoxin G2, From Aspergillus Flavus, >=98.0% (hplc/tlc)
30. Aflatoxin G2 Solution, 0.5 Mug/ml In Acetonitrile, Analytical Standard
31. Aflatoxin G2 Solution, 3.80 Mug/g In Acetonitrile, Erm(r) Certified Reference Material
32. (3s,7r)-11-methoxy-6,8,16,20-tetraoxapentacyclo[10.8.0.02,9.03,7.013,18]icosa-1,9,11,13(18)-tetraene-17,19-dione
33. (7ar,cis)3,4,7a,9,10,10a-hexahydro-5-methoxy-1h,12h- Furo(3',2':4,5)furo(2,3-h)pyrano(3,4-c)chromene-1,12-dione
34. (7ar,cis)3,4,7a,9,10,10a-hexahydro-5-methoxy-1h,12h-furo(3',2':4,5)furo(2,3-h)pyrano(3,4-c)chromene-1,12-dione
35. 1h,12h-furo(3',2':4,5)furo(2,3-h)pyrano(3,4-c)(1)benzopyran- 1,12-dione, 3,4,7a,9,10,10a-hexahydro-5-methoxy-, (7ar,10as)-
36. 1h,12h-furo(3',2':4,5)furo(2,3-h)pyrano(3,4-c)(1)benzopyran-1,12-dione, 3,4,7a-alpha,9,10,10a-alpha-hexahydro-5-methoxy-
37. Aflatoxin G2 Solution, Certified Reference Material, 3 Mug/ml In Benzene:acetonitrile (98:2), Ampule Of 1 Ml
| Molecular Weight | 330.29 g/mol |
|---|---|
| Molecular Formula | C17H14O7 |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 1 |
| Exact Mass | 330.07395278 g/mol |
| Monoisotopic Mass | 330.07395278 g/mol |
| Topological Polar Surface Area | 80.3 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 626 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
To evaluate the rate at which the four main aflatoxins (aflatoxins B1, B2, G1 and G2) are able to cross the luminal membrane of the rat small intestine, a study about intestinal absorption kinetics of these mycotoxins has been made. In situ results obtained showed that the absorption of aflatoxins in rat small intestine is a very fast process that follows first-order kinetics, with an absorption rate constant (ka) of 5.84 +/- 0.05 (aflatoxin B1), 4.06 +/- 0.09 (aflatoxin B2), 2.09 +/- 0.03 (aflatoxin G1) and 1.58 +/- 0.04 (aflatoxin G2) h-1, respectively.
Ramos A et al; Mycopathologia 134(1): p.27-30 (1996)
Aflatoxin G2 ... /is/ the 9,10-dihydro derivative of aflatoxin G1.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 35
With the 4 principal Aflatoxins tested, the order of inhibitory effect on RNA polymerase II was: B1 greater than G1 greater than B1, G2.
Yu FL et al; Carcinogenesis (London) 3 (9): 1005 (1982)
Phagocytosis, intracellular killing of Candida albicans, and superoxide production by rat peritoneal macrophages exposed to aflatoxins B1, B2, G1, G2, B2a, and M1 at several times and concentration were analyzed to evaluate the intensity of a depressive effect for each mycotoxin. All aflatoxins used at very low concn had a depressive effect on the functions of macrophages. The biggest impairment of phagocytosis, intracellular killing, and spontaneous superoxide production was observed in macrophages exposed to aflatoxins B1 and M1.
PMID:2176448 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC184993 Cusumano V et al; Appl Environ Microbiol 56 (11): 3482-4 (1990)


