
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
VMF
0
Canada
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
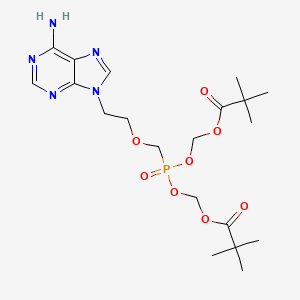

1. 9-(2-((-bis((pivaloyloxy)methoxy)phosphinyl)methoxy)ethyl)adenine
2. Adefovir Depivoxil
3. Gs 840
4. Gs-0840
5. Hepsera
6. Preveon
1. 142340-99-6
2. Hepsera
3. Preveon
4. Adefovir Pivoxil
5. Bis-pom Pmea
6. Gs-0840
7. Gs 0840
8. Gs 840
9. Gs-840
10. [2-(6-aminopurin-9-yl)ethoxymethyl-(2,2-dimethylpropanoyloxymethoxy)phosphoryl]oxymethyl 2,2-dimethylpropanoate
11. Adefovir Dipivoxil [usan]
12. Adefovir Pivoxil (jan)
13. Adefovir Di(pivaloyloxymethyl) Ester
14. Adefovir Dipivoxil (usan)
15. 9-(2-((bis((pivaloyloxy)methoxy)phosphinyl)methoxy)ethyl)adenine
16. (((2-(6-amino-9h-purin-9-yl)ethoxy)methyl)phosphinylidene)bis(oxymethylene) 2,2-dimethylpropanoate
17. ((2-(6-amino-9h-purin-9-yl)ethoxy)methyl)phosphonic Acid, Diester With Hydroxymethyl Pivalate
18. Adefovir Dipivoxil (preveon, Hepsera)
19. U6q8z01514
20. Propanoic Acid, 2,2-dimethyl-, (((2-(6-amino-9h-purin-9-yl)ethoxy)methyl)phosphinylidene)bis(oxymethylene) Ester
21. Ncgc00164624-01
22. Adefovirdipivoxl
23. Dsstox_cid_26487
24. Dsstox_rid_81658
25. Dsstox_gsid_46487
26. ((((2-(6-amino-9h-purin-9-yl)ethoxy)methyl)phosphoryl)bis(oxy))bis(methylene) Bis(2,2-dimethylpropanoate)
27. Adefovir Pivoxil [jan]
28. 9-[2-[bis(pivaloyloxymethoxy)phosphorylmethoxy]ethyl]adenine
29. Youheding
30. Adefovir Dipivoxyl
31. [({[2-(6-amino-9h-purin-9-yl)ethoxy]methyl}({[(2,2-dimethylpropanoyl)oxy]methoxy})phosphoryl)oxy]methyl 2,2-dimethylpropanoate
32. Bis(pom)pmea
33. Smr002530072
34. Hepsera (tm)
35. Hepsera (tn)
36. Piv2pmea
37. Cas-142340-99-6
38. Bis(pom)-pmea
39. 9-(2-((-bis((pivaloyloxy)methoxy)phosphinyl)methoxy)ethyl)adenine
40. Unii-u6q8z01514
41. Adefovir-dipivoxil
42. Mfcd00869897
43. Adefovir Dipivoxil- Bio-x
44. Chembl922
45. Schembl29729
46. Mls003915635
47. Mls004774124
48. Dtxsid5046487
49. Chebi:31175
50. 9-(2[bis(pivaloyloxymethoxy)phosphorylmethoxy]ethyl)adenine
51. Bis(pivaloyloxymethyl)-9-(2-phosphonylmethoxyethyl)adenine
52. Adefovir Dipivoxil [vandf]
53. Bcpp000434
54. Hms3604f09
55. Hms3655i04
56. Adefovir Dipivoxil [mart.]
57. Adefovir Dipivoxil [who-dd]
58. Bcp22959
59. Hy-b0255
60. Zinc3930376
61. Tox21_112243
62. Bdbm50248359
63. S1718
64. Stl257088
65. Adefovir Dipivoxil [ema Epar]
66. Akos005145737
67. Tox21_112243_1
68. Ab07592
69. Ac-1693
70. Bcp9000232
71. Ccg-269700
72. Db00718
73. Gs-3604
74. Adefovir Dipivoxil [orange Book]
75. Ncgc00164624-02
76. Ncgc00164624-03
77. Ncgc00164624-11
78. Ba164139
79. Bcp0726000157
80. B2222
81. Ft-0631164
82. Sw219933-1
83. D01655
84. Ab01274706-01
85. Ab01274706_02
86. 340a996
87. A807894
88. Adefovir Di(pivaloyloxymethyl) Ester [mi]
89. Adefovir Dipivoxil 100 Microg/ml In Acetonitrile
90. Sr-01000931258
91. Q-101927
92. Sr-01000931258-2
93. Brd-k96159438-001-03-0
94. Adefovir Dipivoxil, Antibiotic For Culture Media Use Only
95. Bis(pivaloyloxymethyl)-9-[2-(phosphonomethoxy)ethyl]adenine
96. ((((2-(6-amino-9h-purin-9-yl)ethoxy)methyl)phosphoryl)-bis(oxy))bis(methylene) Bis(2,2-dimethylpropanoate)
97. (((2-(6-amino-9h-purin-9-yl)ethoxy)methyl)phosphoryl)bis(oxy)bis(methylene) Bis(2,2-dimethylpropanoate)
98. ({[2-(6-amino-9h-purin-9-yl)ethoxy]methyl}phosphoryl)bis(oxymethanediyl) Bis(2,2-dimethylpropanoate)
99. 2,2-dimethylpropanoic Acid [2-(6-aminopurin-9-yl)ethoxymethyl-[(2,2-dimethyl-1-oxopropoxy)methoxy]phosphoryl]oxymethyl Ester;adefovir Dipivoxyl
100. 3-{[2-(6-amino-9h-purin-9-yl)ethoxy]methyl}-8,8-dimethyl-3-oxido-7-oxo-2,4,6-trioxa-3?5-phosphanon-1-yl 2,2-dimethylpropanoate
101. 3-{[2-(6-amino-9h-purin-9-yl)ethoxy]methyl}-8,8-dimethyl-3-oxido-7-oxo-2,4,6-trioxa-3lambda~5~-phosphanon-1-yl 2,2-dimethylpropanoate
| Molecular Weight | 501.5 g/mol |
|---|---|
| Molecular Formula | C20H32N5O8P |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 15 |
| Exact Mass | 501.19884999 g/mol |
| Monoisotopic Mass | 501.19884999 g/mol |
| Topological Polar Surface Area | 167 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 706 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Adefovir dipivoxil |
| PubMed Health | Adefovir Dipivoxil (By mouth) |
| Drug Classes | Antiviral |
| Drug Label | HEPSERA is the tradename for adefovir dipivoxil, a diester prodrug of adefovir. Adefovir is an acyclic nucleotide analog with activity against human hepatitis B virus (HBV). The chemical name of adefovir dipivoxil is 9-[2-[[bis[(pivaloyloxy)methoxy... |
| Active Ingredient | Adefovir dipivoxil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Sigmapharm Labs |
| 2 of 4 | |
|---|---|
| Drug Name | Hepsera |
| PubMed Health | Adefovir Dipivoxil (By mouth) |
| Drug Classes | Antiviral |
| Drug Label | HEPSERA is the tradename for adefovir dipivoxil, a diester prodrug of adefovir. Adefovir is an acyclic nucleotide analog with activity against human hepatitis B virus (HBV). The chemical name of adefovir dipivoxil is 9-[2-[[bis[(pivaloyloxy)methoxy... |
| Active Ingredient | Adefovir dipivoxil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Gilead |
| 3 of 4 | |
|---|---|
| Drug Name | Adefovir dipivoxil |
| PubMed Health | Adefovir Dipivoxil (By mouth) |
| Drug Classes | Antiviral |
| Drug Label | HEPSERA is the tradename for adefovir dipivoxil, a diester prodrug of adefovir. Adefovir is an acyclic nucleotide analog with activity against human hepatitis B virus (HBV). The chemical name of adefovir dipivoxil is 9-[2-[[bis[(pivaloyloxy)methoxy... |
| Active Ingredient | Adefovir dipivoxil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Sigmapharm Labs |
| 4 of 4 | |
|---|---|
| Drug Name | Hepsera |
| PubMed Health | Adefovir Dipivoxil (By mouth) |
| Drug Classes | Antiviral |
| Drug Label | HEPSERA is the tradename for adefovir dipivoxil, a diester prodrug of adefovir. Adefovir is an acyclic nucleotide analog with activity against human hepatitis B virus (HBV). The chemical name of adefovir dipivoxil is 9-[2-[[bis[(pivaloyloxy)methoxy... |
| Active Ingredient | Adefovir dipivoxil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Gilead |
Indicated for the treatment of chronic hepatitis B in adult patients with evidence of active viral replication and either evidence of persistent elevations in serum aminotransferases (ALT or AST) or histologically active disease; this is based on histological, virological, biochemical, and serological responses in adult patients with HBeAg+ and HBeAg- chronic hepatitis B with compensated liver function, and in adult patients with clinical evidence of lamivudine-resistant hepatitis B virus with either compensated or decompensated liver function.
FDA Label
Hepsera is indicated for the treatment of chronic hepatitis B in adults with:
- compensated liver disease with evidence of active viral replication, persistently elevated serum-alanine-aminotransferase (ALT) levels and histological evidence of active liver inflammation and fibrosis. Initiation of Hepsera treatment should only be considered when the use of an alternative antiviral agent with a higher genetic barrier to resistance is not available or appropriate (see section 5. 1);
- decompensated liver disease in combination with a second agent without cross-resistance to Hepsera.
Adefovir dipivoxil a diester prodrug of adefovir. Adefovir is an acyclic nucleotide analog with activity against human hepatitis B virus (HBV). The concentration of adefovir that inhibited 50% of viral DNA synthesis (IC50) in vitro ranged from 0.2 to 2.5 μM in HBV transfected human hepatoma cell lines. The combination of adefovir with lamivudine showed additive anti-HBV activity.
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
Reverse Transcriptase Inhibitors
Inhibitors of reverse transcriptase (RNA-DIRECTED DNA POLYMERASE), an enzyme that synthesizes DNA on an RNA template. (See all compounds classified as Reverse Transcriptase Inhibitors.)
J05AF08
J05AF08
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AF - Nucleoside and nucleotide reverse transcriptase inhibitors
J05AF08 - Adefovir dipivoxil
Absorption
The approximate oral bioavailability of adefovir from HEPSERA is 59%. When a single oral 10 mg dose is given to chronic hepatitis B patients, the peak plasma concentration (Cmax) of adefovir was 18.4 6.26 ng/mL. This occurred between 0.58 - 4 hours post dose (Tmax). The adefovir area under the plasma concentration-time curve (AUC0) was 220 70.0 ngh/mL. Food does not affect the exposure of adeforvir.
Route of Elimination
Adefovir is renally excreted by a combination of glomerular filtration and active tubular secretion.
Volume of Distribution
392 75 mL/kg [Vd at steady state, intravenous administration of 1.0 mg/kg/day]
352 9 mL/kg [Vd at steady state, intravenous administration of 3.0 mg/kg/day]
Clearance
469 99.0 mL/min [Patients with Unimpaired renal Function receiving a 10 mg single dose]
356 85.6 mL/min [Patients with mild renal impairement receiving a 10 mg single dose]
237 118 mL/min [Patients with moderate renal impairement receiving a 10 mg single dose]
91.7 51.3 mL/min [Patients with severe renal impairement receiving a 10 mg single dose]
Following oral administration, adefovir dipivoxil is rapidly converted to adefovir. 45% of the dose is recovered as adefovir in the urine over 24 hours at steady state following 10 mg oral doses. Adefovir is not a substrate of the cytochrome P450 enzymes.
Plasma adefovir concentrations declined in a biexponential manner with a terminal elimination half-life of 7.48 1.65 hours.
Adefovir dipivoxil is a prodrug of adefovir. Adefovir is an acyclic nucleotide analog of adenosine monophosphate which is phosphorylated to the active metabolite adefovir diphosphate by cellular kinases. Adefovir diphosphate inhibits HBV DNA polymerase (reverse transcriptase) by competing with the natural substrate deoxyadenosine triphosphate and by causing DNA chain termination after its incorporation into viral DNA. The inhibition constant (Ki) for adefovir diphosphate for HBV DNA polymerase was 0.1 μM. Adefovir diphosphate is a weak inhibitor of human DNA polymerases α and γ with Ki values of 1.18 μM and 0.97μM, respectively.
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
89
PharmaCompass offers a list of Adefovir Dipivoxil API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Adefovir Dipivoxil manufacturer or Adefovir Dipivoxil supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Adefovir Dipivoxil manufacturer or Adefovir Dipivoxil supplier.
PharmaCompass also assists you with knowing the Adefovir Dipivoxil API Price utilized in the formulation of products. Adefovir Dipivoxil API Price is not always fixed or binding as the Adefovir Dipivoxil Price is obtained through a variety of data sources. The Adefovir Dipivoxil Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Adefovir Dipivoxil manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Adefovir Dipivoxil, including repackagers and relabelers. The FDA regulates Adefovir Dipivoxil manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Adefovir Dipivoxil API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Adefovir Dipivoxil manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Adefovir Dipivoxil supplier is an individual or a company that provides Adefovir Dipivoxil active pharmaceutical ingredient (API) or Adefovir Dipivoxil finished formulations upon request. The Adefovir Dipivoxil suppliers may include Adefovir Dipivoxil API manufacturers, exporters, distributors and traders.
click here to find a list of Adefovir Dipivoxil suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Adefovir Dipivoxil DMF (Drug Master File) is a document detailing the whole manufacturing process of Adefovir Dipivoxil active pharmaceutical ingredient (API) in detail. Different forms of Adefovir Dipivoxil DMFs exist exist since differing nations have different regulations, such as Adefovir Dipivoxil USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Adefovir Dipivoxil DMF submitted to regulatory agencies in the US is known as a USDMF. Adefovir Dipivoxil USDMF includes data on Adefovir Dipivoxil's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Adefovir Dipivoxil USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Adefovir Dipivoxil suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Adefovir Dipivoxil Drug Master File in Korea (Adefovir Dipivoxil KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Adefovir Dipivoxil. The MFDS reviews the Adefovir Dipivoxil KDMF as part of the drug registration process and uses the information provided in the Adefovir Dipivoxil KDMF to evaluate the safety and efficacy of the drug.
After submitting a Adefovir Dipivoxil KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Adefovir Dipivoxil API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Adefovir Dipivoxil suppliers with KDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Adefovir Dipivoxil as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Adefovir Dipivoxil API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Adefovir Dipivoxil as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Adefovir Dipivoxil and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Adefovir Dipivoxil NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Adefovir Dipivoxil suppliers with NDC on PharmaCompass.
Adefovir Dipivoxil Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Adefovir Dipivoxil GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Adefovir Dipivoxil GMP manufacturer or Adefovir Dipivoxil GMP API supplier for your needs.
A Adefovir Dipivoxil CoA (Certificate of Analysis) is a formal document that attests to Adefovir Dipivoxil's compliance with Adefovir Dipivoxil specifications and serves as a tool for batch-level quality control.
Adefovir Dipivoxil CoA mostly includes findings from lab analyses of a specific batch. For each Adefovir Dipivoxil CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Adefovir Dipivoxil may be tested according to a variety of international standards, such as European Pharmacopoeia (Adefovir Dipivoxil EP), Adefovir Dipivoxil JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Adefovir Dipivoxil USP).

