
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
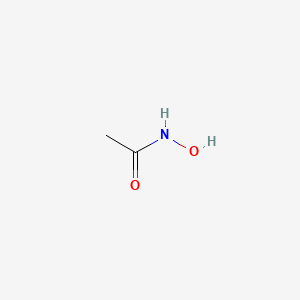

1. Lithostat
2. N-hydroxyacetamide
3. N-hydroxyacetamidine
4. Uronefrex
1. N-hydroxyacetamide
2. 546-88-3
3. Lithostat
4. Methylhydroxamic Acid
5. Acetylhydroxamic Acid
6. Acetic Acid, Oxime
7. N-acetylhydroxylamine
8. Acetohydroximic Acid
9. Acetamide, N-hydroxy-
10. Acethydroxamsaeure
11. N-acetyl Hydroxyacetamide
12. Cetohyroxamic Acid
13. Acetohydroxamate
14. Acethydroxamsaure
15. Acethydroxamic Acid
16. Aha
17. Hydroxylamine, N-acetyl-
18. Acido Acetohidroxamico
19. Acide Acetohydroxamique
20. Acidum Acetohydroxamicum
21. N-hydroxy-acetamide
22. Nsc 176136
23. Mfcd00009994
24. Nsc-176136
25. 4rz82l2gy5
26. Acetamide, N-hydroxy- (9ci)
27. Chebi:27777
28. Ncgc00094576-01
29. Dsstox_cid_2546
30. Wln: Qmv1
31. Acetohydroxamicacid
32. Acetyl Hydroxyamino
33. Dsstox_rid_76622
34. Dsstox_gsid_22546
35. Acethydroxamsaeure [german]
36. Oxime
37. Acide Acetohydroxamique [french]
38. Acido Acetohidroxamico [spanish]
39. Acidum Acetohydroxamicum [latin]
40. Lithostat (tn)
41. Cas-546-88-3
42. Ccris 1730
43. Hsdb 3585
44. N-hydroxyacetimidic Acid
45. N-hydroxyethanimidic Acid
46. Einecs 208-913-8
47. Unii-4rz82l2gy5
48. Ai3-62232
49. Acetohydroxamsaure
50. Acetohydroxamic Acid (usp/inn)
51. Acetic Acid
52. Oxime
53. N-oxylatoacetamide
54. Acethydroximic Acid
55. Acetohyroxamic Acid
56. Acetyl Hydroxyamine
57. Prestwick_38
58. Acetohydroxamic-acid
59. N-oxidanylethanamide
60. Acetohydroxarnic Acid
61. Acetohydroxamic Acid [usan:usp:inn]
62. Methyl Hydroximic Acid
63. Spectrum_000020
64. Spectrum2_000109
65. Spectrum3_000285
66. Spectrum4_000138
67. Spectrum5_000812
68. Ch3c(o)nhoh
69. Chembl734
70. Acetohydroxamic Acid, 98%
71. Bspbio_001790
72. Kbiogr_000556
73. Kbioss_000360
74. Mls001076662
75. Divk1c_000821
76. Spectrum1500103
77. Spbio_000098
78. Dtxsid7022546
79. Chebi:49029
80. Hms502j03
81. Kbio1_000821
82. Kbio2_000360
83. Kbio2_002928
84. Kbio2_005496
85. Kbio3_001290
86. Acetohydroxamic Acid [mi]
87. Nsc5073
88. Ninds_000821
89. Acetohydroxamic Acid (aha)
90. Acetohydroxamic Acid [inn]
91. Hms1920a07
92. Hms2091g07
93. Hms2231m17
94. Pharmakon1600-01500103
95. Acetohydroxamic Acid [hsdb]
96. Acetohydroxamic Acid [usan]
97. Act05768
98. Hy-b1235
99. Nsc-5073
100. Str08084
101. Zinc4658603
102. Acetohydroxamic Acid [vandf]
103. Tox21_111301
104. Acetohydroxamic Acid [mart.]
105. Bdbm50099857
106. Ccg-38927
107. Geo-00010
108. Nsc176136
109. Nsc755855
110. S4602
111. Acetohydroxamic Acid [usp-rs]
112. Acetohydroxamic Acid [who-dd]
113. Akos000172340
114. Tox21_111301_1
115. Ab01014
116. Cs-4881
117. Db00551
118. Nsc-755855
119. Idi1_000821
120. Ncgc00094576-02
121. Ncgc00094576-03
122. Ncgc00094576-05
123. Acetohydroxamic Acid [orange Book]
124. Bp-13320
125. Smr000499570
126. Sbi-0051270.p003
127. Acetohydroxamic Acid [usp Monograph]
128. Db-052632
129. A0051
130. Am20100343
131. Ft-0621796
132. En300-36948
133. 46a883
134. C06808
135. D00220
136. Ab00051907_07
137. A830321
138. Q481822
139. Sr-01000763642
140. Sr-01000763642-2
141. W-105609
142. Acetohydroxamic Acid, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 75.07 g/mol |
|---|---|
| Molecular Formula | C2H5NO2 |
| XLogP3 | -1.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 75.032028402 g/mol |
| Monoisotopic Mass | 75.032028402 g/mol |
| Topological Polar Surface Area | 49.3 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 42.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Lithostat |
| PubMed Health | Acetohydroxamic Acid (By mouth) |
| Drug Classes | Urinary Stone Agent |
| Drug Label | Acetohydroxamic acid (AHA) is a stable, synthetic compound derived from hydroxylamine and ethyl acetate. Its molecular structure is similar to urea:AHA is weakly acidic, highly soluble in water, and chelates metals - notably iron. The molecular weigh... |
| Active Ingredient | Acetohydroxamic acid |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg |
| Market Status | Prescription |
| Company | Mission Pharma |
| 2 of 2 | |
|---|---|
| Drug Name | Lithostat |
| PubMed Health | Acetohydroxamic Acid (By mouth) |
| Drug Classes | Urinary Stone Agent |
| Drug Label | Acetohydroxamic acid (AHA) is a stable, synthetic compound derived from hydroxylamine and ethyl acetate. Its molecular structure is similar to urea:AHA is weakly acidic, highly soluble in water, and chelates metals - notably iron. The molecular weigh... |
| Active Ingredient | Acetohydroxamic acid |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg |
| Market Status | Prescription |
| Company | Mission Pharma |
Enzyme Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Acetohydroxamic acid is indicated in the prophylaxis of struvite calculi formation that is promoted by urease-producing bacteria such as Proteus. Its use may enhance effectiveness of urinary antibacterials, especially following surgical removal of existing stones. Use of acetohydroxamic acid also improves the possibility of reducing the frequency and rate of new stone formation. /Included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 17th ed. Volume I. Rockville, MD: Convention, Inc., 1997. (Plus Updates)., p. 14
Acetohydroxamic acid is indicated as an adjunct in the treatment of chronic, urea-splitting urinary tract infections caused by urease-producing bacteria. Its inhibition of urease activity decreases the urinary ammonia and alkalinity produced from the enzyme hydrolysis of urea. /Included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 17th ed. Volume I. Rockville, MD: Convention, Inc., 1997. (Plus Updates)., p. 14
Acetohydroxamic acid is not indicated for dissolution of existing calculi, replacement of indicated surgical treatment, urinary tract infections controllable by culture-specific oral antibacterials, or urinary tract infections caused by nonurease producing organisms.
USP Convention. USPDI - Drug Information for the Health Care Professional. 17th ed. Volume I. Rockville, MD: Convention, Inc., 1997. (Plus Updates)., p. 14
Use of acetohydroxamic acid is contraindicated during pregnancy since studies in animals have shown it to cause leg deformities at doses of 750 mg/kg of body weight and above. At doses of 1500 mg/kg, exencephaly and encephalocele occurred. Also, cardiac, coccygeal, and abdominal-wall anomalies developed in pups of beagle dogs given 25 mg/kg a day during pregnancy.
USP Convention. USPDI - Drug Information for the Health Care Professional. 17th ed. Volume I. Rockville, MD: Convention, Inc., 1997. (Plus Updates)., p. 14
It is not known if acetohydroxamic acid is distributed into breast milk. Although problems in humans have not been documented, its use is not recommended in breast-feeding mothers because of the potential for serious adverse effects in the nursing infant.
USP Convention. USPDI - Drug Information for the Health Care Professional. 17th ed. Volume I. Rockville, MD: Convention, Inc., 1997. (Plus Updates)., p. 14
Headache, appearing during the first 48 hours of treatment, reportedly occurs in approximately 30% of patients receiving acetohydroxamic acid; however, several clinicians reported that mild, transient headache occurred in 70-75% of patients during initiation of therapy. Headache is generally mild, responsive to oral salicylate analgesics, and usually disappears spontaneously. Headache has not been associated with vertigo, tinnitus, or visual or auditory disturbances. Malaise occurs in about 20-25% of patients receiving the drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 97. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1997 (Plus Supplements)., p. 1980
For more Drug Warnings (Complete) data for ACETOHYDROXAMIC ACID (12 total), please visit the HSDB record page.
Used, in addition to antibiotics or medical procedures, to treat chronic urea-splitting urinary infections.
Acetohydroxamic Acid, a synthetic drug derived from hydroxylamine and ethyl acetate, is similar in structure to urea. In the urine, it acts as an antagonist of the bacterial enzyme urease. Acetohydroxamic Acid has no direct antimicrobial action and does not acidify urine directly.
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
G - Genito urinary system and sex hormones
G04 - Urologicals
G04B - Urologicals
G04BX - Other urologicals
G04BX03 - Acetohydroxamic acid
Absorption
Well absorbed from the GI tract following oral administration.
Well absorbed from gastrointestinal tract.
USP Convention. USPDI - Drug Information for the Health Care Professional. 17th ed. Volume I. Rockville, MD: Convention, Inc., 1997. (Plus Updates)., p. 14
Well distributed throughout body fluids.
USP Convention. USPDI - Drug Information for the Health Care Professional. 17th ed. Volume I. Rockville, MD: Convention, Inc., 1997. (Plus Updates)., p. 14
Elimination: Renal- Unchanged, 36 to 65%; as acetamide, 9 to 14%. Respiratory - As carbon dioxide, 20 to 40%.
USP Convention. USPDI - Drug Information for the Health Care Professional. 17th ed. Volume I. Rockville, MD: Convention, Inc., 1997. (Plus Updates)., p. 14
In rodents, about 55% of an intraperitoneal dose is excreted in urine as unchanged drug, 15% as acetamide, and 10% as acetate within 24 hours; approximately 7% of the dose is excreted by the lungs as carbon dioxide and less than 1% is excreted in feces within 24 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 97. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1997 (Plus Supplements)., p. 1979
In mice, highest concentrations of the drug occur in the liver and kidney, while the lowest concentrations occur in the brain.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 97. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1997 (Plus Supplements)., p. 1978
35-65% of oral dose excreted unchanged in urine (which provides the drug's therapeutic effect).
...is metabolized to acetamide.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 97. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1997 (Plus Supplements)., p. 1979
5-10 hours in patients with normal renal function
...increases with increasing dose and reportedly ranges from about 3.5-10 hours in patients with normal renal function.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 97. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1997 (Plus Supplements)., p. 1979
Acetohydroxamic Acid reversibly inhibits the bacterial enzyme urease. This inhibits the hydrolysis of urea and production of ammonia in urine infected with urea-splitting organisms, leading to a decrease in pH and ammonia levels. As antimicrobial agents are more effective in such conditions, the effectiveness of these agents is amplified, resulting in a higher cure rate.
Inhibits the hydrolysis of urea and production of ammonia in urine infected with urea-splitting bacteria, by reversible inhibition of the bacterial enzyme urease, and by the chelation of nickel, an essential component of urease enzymes. Such enzyme inhibition results in reduction of both urine alkalinity and ammonia concentration. The effectiveness of antibacterial medication is then enhanced and the formation of urinary calculi reduced.
USP Convention. USPDI - Drug Information for the Health Care Professional. 17th ed. Volume I. Rockville, MD: Convention, Inc., 1997. (Plus Updates)., p. 14

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
46
PharmaCompass offers a list of Acetohydroxamic Acid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Acetohydroxamic Acid manufacturer or Acetohydroxamic Acid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Acetohydroxamic Acid manufacturer or Acetohydroxamic Acid supplier.
PharmaCompass also assists you with knowing the Acetohydroxamic Acid API Price utilized in the formulation of products. Acetohydroxamic Acid API Price is not always fixed or binding as the Acetohydroxamic Acid Price is obtained through a variety of data sources. The Acetohydroxamic Acid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Acetohydroxamic Acid manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Acetohydroxamic Acid, including repackagers and relabelers. The FDA regulates Acetohydroxamic Acid manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Acetohydroxamic Acid API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Acetohydroxamic Acid manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Acetohydroxamic Acid supplier is an individual or a company that provides Acetohydroxamic Acid active pharmaceutical ingredient (API) or Acetohydroxamic Acid finished formulations upon request. The Acetohydroxamic Acid suppliers may include Acetohydroxamic Acid API manufacturers, exporters, distributors and traders.
click here to find a list of Acetohydroxamic Acid suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Acetohydroxamic Acid DMF (Drug Master File) is a document detailing the whole manufacturing process of Acetohydroxamic Acid active pharmaceutical ingredient (API) in detail. Different forms of Acetohydroxamic Acid DMFs exist exist since differing nations have different regulations, such as Acetohydroxamic Acid USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Acetohydroxamic Acid DMF submitted to regulatory agencies in the US is known as a USDMF. Acetohydroxamic Acid USDMF includes data on Acetohydroxamic Acid's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Acetohydroxamic Acid USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Acetohydroxamic Acid suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Acetohydroxamic Acid as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Acetohydroxamic Acid API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Acetohydroxamic Acid as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Acetohydroxamic Acid and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Acetohydroxamic Acid NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Acetohydroxamic Acid suppliers with NDC on PharmaCompass.
Acetohydroxamic Acid Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Acetohydroxamic Acid GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Acetohydroxamic Acid GMP manufacturer or Acetohydroxamic Acid GMP API supplier for your needs.
A Acetohydroxamic Acid CoA (Certificate of Analysis) is a formal document that attests to Acetohydroxamic Acid's compliance with Acetohydroxamic Acid specifications and serves as a tool for batch-level quality control.
Acetohydroxamic Acid CoA mostly includes findings from lab analyses of a specific batch. For each Acetohydroxamic Acid CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Acetohydroxamic Acid may be tested according to a variety of international standards, such as European Pharmacopoeia (Acetohydroxamic Acid EP), Acetohydroxamic Acid JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Acetohydroxamic Acid USP).

