
Synopsis
Synopsis
0
CEP/COS
0
EU WC
0
KDMF
0
VMF
0
FDA Orange Book
0
Australia
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Taufon
2. Tauphon
3. Taurine Hydrochloride
4. Taurine Zinc Salt (2:1)
5. Taurine, Monopotassium Salt
1. 2-aminoethanesulfonic Acid
2. 107-35-7
3. Ethanesulfonic Acid, 2-amino-
4. L-taurine
5. Tauphon
6. 2-aminoethylsulfonic Acid
7. 2-sulfoethylamine
8. 2-aminoethane-1-sulfonic Acid
9. O-due
10. Aminoethylsulfonic Acid
11. Taufon
12. Aminoethanesulfonic Acid
13. Beta-aminoethylsulfonic Acid
14. Taurinum [latin]
15. Taurina [spanish]
16. Fema No. 3813
17. Taurine [inn]
18. Ccris 4721
19. Nci-c60606
20. Ai3-18307
21. Nsc32428
22. Nsc-32428
23. 1eqv5mly3d
24. 2-aminoethane Sulfonic Acid
25. .beta.-aminoethylsulfonic Acid
26. 1-aminoethane-2-sulfonic Acid
27. Chebi:15891
28. Ncgc00015997-06
29. Dsstox_cid_1304
30. Dsstox_rid_76069
31. Dsstox_gsid_21304
32. Aminoethylsulfonic Acid (jan)
33. Taurine Hydrochloride
34. 2-amino-ethanesulfonic Acid
35. Taurina
36. Taurinum
37. Aminoethylsulfonic Acid [jan]
38. Cas-107-35-7
39. Smr000326743
40. Sr-01000076144
41. 2-aminoethanesulfonicacid
42. Einecs 203-483-8
43. Unii-1eqv5mly3d
44. Nsc 32428
45. Taurine [usp:inn:ban]
46. Taurineold
47. Taukard
48. Taurate
49. Hsdb 8167
50. Aminoethylsulfonate
51. Taurine,(s)
52. Taurine (tn)
53. B-aminoethylsulfonate
54. Mfcd00008197
55. Taurine (8ci)
56. 2-aminoethylsulfonate
57. 2-aminoethyl Sulfonate
58. Beta-aminoethylsulfonate
59. Taurine-[13c2]
60. Tocris-0209
61. Taurine [vandf]
62. 2aminoethanesulfonic Acid
63. Taurine [fhfi]
64. Taurine [inci]
65. Taurine, >=99%
66. B-aminoethylsulfonic Acid
67. Taurine [jan]
68. Taurine [mi]
69. Taurine [mart.]
70. Lopac-t-0625
71. Taurine [usp-rs]
72. Taurine [who-dd]
73. Wln: Z2swq
74. 1-aminoethane-2-sulfonate
75. Bmse000120
76. Bmse000805
77. Bmse000863
78. Ec 203-483-8
79. 2-aminoethanesulfonic Acid.
80. Lopac0_001134
81. Schembl23068
82. Taurine, >=98%, Fg
83. Mls000859681
84. Mls001332383
85. Mls001332384
86. Taurine (jp17/usp/inn)
87. Chembl239243
88. Gtpl2379
89. Taurine [usp Monograph]
90. Dtxsid3021304
91. Taurine, >=99.0% (t)
92. Hms2093l13
93. Hms2233d19
94. Hms3263d09
95. Hms3370j18
96. Pharmakon1600-01505463
97. Hy-b0351
98. Rkl10149
99. Taurine; 2-aminoethanesulfonic Acid
100. Zinc3809490
101. Tox21_110277
102. Tox21_202520
103. Tox21_501134
104. Bdbm50357220
105. Nsc759150
106. S2008
107. Stl197941
108. Akos005208848
109. Tox21_110277_1
110. Ccg-205208
111. Db01956
112. Ethanesulfonic Acid, 2-amino- (9ci)
113. Lp01134
114. Nsc-759150
115. Sdccgsbi-0051101.p003
116. Taurine, Bioultra, >=99.5% (t)
117. Taurine, Saj First Grade, >=98.5%
118. Ncgc00015997-01
119. Ncgc00015997-02
120. Ncgc00015997-03
121. Ncgc00015997-04
122. Ncgc00015997-05
123. Ncgc00015997-07
124. Ncgc00015997-08
125. Ncgc00015997-10
126. Ncgc00015997-20
127. Ncgc00024497-01
128. Ncgc00024497-02
129. Ncgc00024497-03
130. Ncgc00024497-04
131. Ncgc00024497-05
132. Ncgc00260069-01
133. Ncgc00261819-01
134. As-13587
135. Nci60_002814
136. Sbi-0051101.p002
137. Taurine, Vetec(tm) Reagent Grade, >=99%
138. A0295
139. Am20080018
140. B1846
141. Eu-0101134
142. Ft-0611241
143. T-130
144. C00245
145. D00047
146. D78121
147. T 0625
148. Ab00443712-07
149. Ab00443712_09
150. Ab00443712_10
151. Q207051
152. J-508042
153. Sr-01000076144-1
154. Sr-01000076144-3
155. Sr-01000076144-5
156. Taurine, Certified Reference Material, Tracecert(r)
157. F2191-0280
158. Z1317839154
159. Taurine, Cell Culture Tested, Meets Usp Testing Specifications
160. Taurine, United States Pharmacopeia (usp) Reference Standard
161. Taurine, Pharmaceutical Secondary Standard; Certified Reference Material
162. Taurine, Pharmagrade, Ajinomoto, Manufactured Under Appropriate Gmp Controls For Pharma Or Biopharmaceutical Production, Suitable For Cell Culture
| Molecular Weight | 125.15 g/mol |
|---|---|
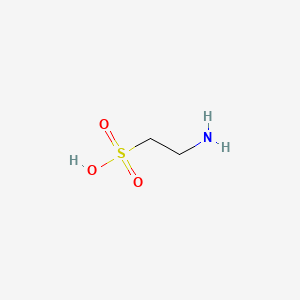
| Molecular Formula | C2H7NO3S |
| XLogP3 | -4.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 125.01466426 g/mol |
| Monoisotopic Mass | 125.01466426 g/mol |
| Topological Polar Surface Area | 88.8 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 120 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Taurine may be helpful in some with congestive heart failure and hypertension. It has demonstrated some antiatherogenic effects in both animal and human studies.
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 607
THERAPEUTIC CATEGORY (VETERINARY): In preventation of retinal degeneration and in prevention and treatment of taurine-deficiency cardiomyopathy in cats
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Cambridge, UK: Royal Society of Chemistry, 2013., p. 1679
Taurine has been added to most human infant formulas since the mid-1980s.
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 607
Pregnant women and nursing mothers should avoid taurine supplements unless recommended by their physicians. Those with congestive heart failure should only use taurine under medical supervision.
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 609
Taurine is contraindicated in those hypersensitive to any component of a taurine-containing nutritional supplement.
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 609
The use of diet supplements containing taurine is indicated for the nutritional support of infants and young pediatric patients requiring total parenteral nutrition via central or peripheral routes. The usage of diet supplements containing taurine prevents nitrogen and weight loss or to treat negative nitrogen balance in pediatric patients where the alimentary tract cannot be done through oral, gastrostomy or jejunostomy administration, there is impaired gastrointestinal absorption or protein requirements are substantially increased.
FDA Label
The diet supplements containing taurine are formulated as a well-tolerated nitrogen source for nutritional support. Administration of diet supplements regulates the level of plasma amino acid concentration, nitrogen balance, weight and serum protein concentration to reach normal values, thus improving the nutritional status.
Absorption
Oral administration of taurine was studied and it reported dose-dependent values of AUC, Cmax and tmax wherein a dose of 1-30 mg/kg ranged from 89-3452 mcg min/L, 2-15.7 mcg min/ml and 15 min respectively. Further studies in healthy individuals gave an AUC, Cmax and tmax in the range of 116-284.5 mg h/L, 59-112.6 mg/L and 1-2.5 h.
Route of Elimination
Taurine flows and gets distributed in veins and arteries and reports have observed the presence of a significant released of taurine in portally drained viscera, thus suggesting that the main elimination route of taurine is by the gut. This elimination route may be explained by the enterohepatic cycle of taurine.
Volume of Distribution
The distribution of taurine was studied under the two-compartment model and each one of the compartments gave a range for the volume of distribution of 299-353 ml/kg in compartment 1 and 4608-8374 ml/kg in compartment 2 in mice. Further studies in healthy indivudals gave a volume of distribution that ranged from 19.8 to 40.7 L.
Clearance
The clearance rate of orally administered taurine was reported to be dose-dependent wherein a dose of 1 mg/kg it presents a clearance rate of 11.7 ml min/kg, 10 mg/kg generates a clearance rate of 18.7 ml min/kg and a dose of 30 mg/kg reports a clearance rate of 9.4 ml min/kg. Further studies in healthy individuals generate a clearance rate that ranged from 14 to 34.4 L/h.
Taurine is not usually completely reabsorbed from the kidneys, and some fraction of an ingested dose of taurine is excreted in the urine.
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 608
Following ingestion, taurine is absorbed from the small intestine via the beta-amino acid or taurine transport system... Tautine is transported to the liver via the portal circulation, where much of it forms conjugates with bile acids... The taurine conjugates are excreted via the biliary route. Taurine that is not conjugated in the liver is distributed via the systemic circulation to various tissues in the body.
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 608
Taurine is present in high amounts in the brain, retina, myocardium, skeletal and smoth muscle, platelets and neutrophils.
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 607
Human studies showed significant increases in plasma taurine 90 minutes after consumption of a taurine-rich meal with levels declining to background within 180-270 minutes...
European Food Safety Authority (EFSA); EFSA Journal 935: 1-31 (2009). Available from, as of August 19, 2014: https://www.efsa.europa.eu/en/efsajournal/doc/935.pdf
For more Absorption, Distribution and Excretion (Complete) data for Taurine (7 total), please visit the HSDB record page.
Taurine can be metabolized by diverse organisms to form different types of metabolites derived from the original form of taurine. In the human, the pathways that form the metabolism of taurine are divided in the formation of 5-glutamyl-taurine by the action of the enzyme gamma-glutamyltransferase 6 or the formation of taurocholate by the action of the bile acid-CoA:amino acid N-acyltransferase.
There are two sources of taurine in the body: dietary and endogenous. In mammals, taurine is synthesised in many tissues; the main sites are liver, brain and pancreas, predominantly in alpha-islets. Taurine is synthesised from cysteine and methionine in a few steps, one of which requires pyridoxal-5-phosphate (vitamin B6) as coenzyme of cysteine sulphinate decarboxylase. In species other than mammals, the biosynthesis of taurine has been poorly studied. The extent of synthesis varies widely between species. An adult rat consuming standard laboratory food produces about 80 % of its total body taurine and obtains the remainder from the diet. However, if required, rats can obtain all body taurine from biosynthesis, since rats fed taurine-free diets for extended periods do not exhibit any decrease in tissue taurine concentrations. Cats have low levels of activity of cysteine sulphinate decarboxylase, the rate-limiting enzyme for taurine biosynthesis, and are, therefore, dependent on a dietary source to maintain their body pool of this amino acid. Thus, taurine is an essential nutrient in cats.
European Food Safety Authority (EFSA); EFSA Journal 10 (6): 2736 (2012). Available from, as of August 19, 2014: https://www.efsa.europa.eu/en/efsajournal/doc/2736.pdf
Taurocholate, the bile salt conjugate of taurine and cholic acid, is the principal conjugate formed via the action of the enzyme choloyl-CoA N-acyltransferase.
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 608
In all vertebrates except mammals, taurine is the sole amino acid conjugated to form bile salts. Among the mammals, carnivores also tend to be conjugators of taurine only, whereas other species tend to conjugate both taurine and glycine. High concentrations of taurine are present in retina, liver, pancreas, central nervous system and white blood cells. The largest pools of taurine are found in skeletal and cardiac muscles, where it regulates intracellular Ca2+ concentration...
European Food Safety Authority (EFSA); EFSA Journal 10 (6): 2736 (2012). Available from, as of August 19, 2014: https://www.efsa.europa.eu/en/efsajournal/doc/2736.pdf
Oral administration of taurine in healthy individuals gave a plasma elimination half-life that ranged from 0.7-1.4 h.
The diet supplements containing taurine function by replacing the missing nutriments in the body. Taurine, as a single agent, presents different functions like substrate for formation of bile salts, cell volume regulation, modulation of intracellular calcium, cytoprotection of central nervous system, etc.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
60
PharmaCompass offers a list of Taurine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Taurine manufacturer or Taurine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Taurine manufacturer or Taurine supplier.
PharmaCompass also assists you with knowing the Taurine API Price utilized in the formulation of products. Taurine API Price is not always fixed or binding as the Taurine Price is obtained through a variety of data sources. The Taurine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A 1-Aminoethane-2-sulfonate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of 1-Aminoethane-2-sulfonate, including repackagers and relabelers. The FDA regulates 1-Aminoethane-2-sulfonate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. 1-Aminoethane-2-sulfonate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of 1-Aminoethane-2-sulfonate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A 1-Aminoethane-2-sulfonate supplier is an individual or a company that provides 1-Aminoethane-2-sulfonate active pharmaceutical ingredient (API) or 1-Aminoethane-2-sulfonate finished formulations upon request. The 1-Aminoethane-2-sulfonate suppliers may include 1-Aminoethane-2-sulfonate API manufacturers, exporters, distributors and traders.
click here to find a list of 1-Aminoethane-2-sulfonate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A 1-Aminoethane-2-sulfonate DMF (Drug Master File) is a document detailing the whole manufacturing process of 1-Aminoethane-2-sulfonate active pharmaceutical ingredient (API) in detail. Different forms of 1-Aminoethane-2-sulfonate DMFs exist exist since differing nations have different regulations, such as 1-Aminoethane-2-sulfonate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A 1-Aminoethane-2-sulfonate DMF submitted to regulatory agencies in the US is known as a USDMF. 1-Aminoethane-2-sulfonate USDMF includes data on 1-Aminoethane-2-sulfonate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The 1-Aminoethane-2-sulfonate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of 1-Aminoethane-2-sulfonate suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The 1-Aminoethane-2-sulfonate Drug Master File in Japan (1-Aminoethane-2-sulfonate JDMF) empowers 1-Aminoethane-2-sulfonate API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the 1-Aminoethane-2-sulfonate JDMF during the approval evaluation for pharmaceutical products. At the time of 1-Aminoethane-2-sulfonate JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of 1-Aminoethane-2-sulfonate suppliers with JDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing 1-Aminoethane-2-sulfonate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for 1-Aminoethane-2-sulfonate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture 1-Aminoethane-2-sulfonate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain 1-Aminoethane-2-sulfonate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a 1-Aminoethane-2-sulfonate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of 1-Aminoethane-2-sulfonate suppliers with NDC on PharmaCompass.
1-Aminoethane-2-sulfonate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of 1-Aminoethane-2-sulfonate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right 1-Aminoethane-2-sulfonate GMP manufacturer or 1-Aminoethane-2-sulfonate GMP API supplier for your needs.
A 1-Aminoethane-2-sulfonate CoA (Certificate of Analysis) is a formal document that attests to 1-Aminoethane-2-sulfonate's compliance with 1-Aminoethane-2-sulfonate specifications and serves as a tool for batch-level quality control.
1-Aminoethane-2-sulfonate CoA mostly includes findings from lab analyses of a specific batch. For each 1-Aminoethane-2-sulfonate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
1-Aminoethane-2-sulfonate may be tested according to a variety of international standards, such as European Pharmacopoeia (1-Aminoethane-2-sulfonate EP), 1-Aminoethane-2-sulfonate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (1-Aminoethane-2-sulfonate USP).

