



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
0
0
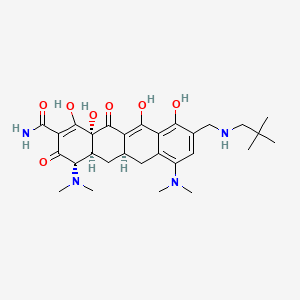

1. Nuzyra
1. Amadacycline
2. 389139-89-3
3. Nuzyra
4. Ptk 0796
5. Omadacycline [usan]
6. Ptk-0796
7. Bay 73-6944
8. 090ip5rv8f
9. 389139-89-3 (free Base)
10. (4s,4as,5ar,12ar)-4,7-bis(dimethylamino)-9-[(2,2-dimethylpropylamino)methyl]-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide
11. Omadacycline (usan)
12. (4s,4as,5ar,12as)-4,7-bis(dimethylamino)-9-(((2,2-dimethylpropyl)amino)methyl)- 3,10,12,12a- Tetrahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2- Carboxamide
13. Amadacycline Methanesulfonate
14. Omadacycline [usan:inn]
15. Unii-090ip5rv8f
16. (4s,4as,5ar,12as)-4,7-bis(dimethylamino)-9-[(2,2-dimethylpropylamino)methyl]-3,10,12,12a-tetrahydroxy-1,11-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide
17. 2-naphthacenecarboxamide, 4,7-bis(dimethylamino)-9-(((2,2-dimethylpropyl)amino)methyl)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-, (4s,4as,5ar,12as)-
18. 2-naphthacenecarboxamide, 4,7-bis(dimethylamino)-9-[[(2,2-dimethylpropyl)amino]methyl]-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-, (4s,4as,5ar,12as)-
19. Mk-2764
20. Omadacycline [mi]
21. Omadacycline [inn]
22. Omadacycline [who-dd]
23. Schembl1525961
24. 9-neopentylaminomethylminocycline
25. Chembl1689772
26. Schembl17150976
27. Schembl20952297
28. Gtpl10839
29. Ptk-796
30. Ptk0796
31. Chebi:177758
32. Dtxsid201027687
33. Bcp12946
34. Ex-a4252
35. Zinc4836283
36. Compound 6 [pmid: 21302930]
37. Cs-1338
38. Db12455
39. Bay-73-6944
40. Bay-73-7388
41. Ncgc00378946-03
42. Ac-33245
43. Hy-14865
44. Ptk 0796, Bay 73-6944
45. D09647
46. E80520
47. Q15426992
48. (4s,4as,5ar,12as)-4,7-bis(dimethylamino)-9-(((2,2-dimethylpropyl)amino)methyl)-3,10,12,12a- Tetrahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide
49. 2-naphthacenecarboxamide, 4,7-bis(dimethylamino)-9-(((2,2- Dimethylpropyl)amino)methyl)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a- Tetrahydroxy-1,11-dioxo-, (4s,4as,5ar,12as)-
| Molecular Weight | 556.6 g/mol |
|---|---|
| Molecular Formula | C29H40N4O7 |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 7 |
| Exact Mass | 556.28969963 g/mol |
| Monoisotopic Mass | 556.28969963 g/mol |
| Topological Polar Surface Area | 177 Ų |
| Heavy Atom Count | 40 |
| Formal Charge | 0 |
| Complexity | 1140 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Omadacycline is indicated for the treatment of community acquired bacterial pneumonia and acute bacterial skin and skin structure infections caused by omadacycline-susceptible organisms in adults.
FDA Label
Treatment of bacterial pneumonia
Treatment of acute bacterial skin and skin structure infections
Omadacycline can be either bacteriostatic or bacteriocidal depending on the organism involved. It disrupts bacterial protein synthesis without affecting DNA, RNA, or peptidoglycan synthesis. Omadacycline represents an improvement over existing tetracycline agents as it has not been found to be subject to tetracycline resistance mediated by tetracycline efflux pumps encoded by the tet(K), tet(L), and tet(B) or to ribosomal protection proteins encoded by tet(O) and tet(M). Omadacycline is susceptible to RNA mutations which confer resistance to tetracyclines.
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01A - Tetracyclines
J01AA - Tetracyclines
J01AA15 - Omadacycline
Absorption
Omadacycline has an mean absolute oral bioavailability of 34.5% and a mean Tmax of2.5 h with oral dosing. With multiple dosing, Omadacycline displays an accumulation factor of 1.5. Official labeling states that food does not significantly impact rate or extent of absorption, however, conflicting data exists suggesting food may lower the bioavailability of omadacycline taken after eating. The exposure in alveolar cells and epithelial lining fluid is 25.8 and 1.5 fold higher than plasma exposure after IV administration, suggesting Omadacycline penetrates the lungs to a significant degree.
Route of Elimination
After IV dosing 27% of Omadacycline was eliminated by the kidneys. In oral dosing 14.4% was found to be eliminated by the kidneys and 81.1% in the feces. Neither renal nor hepatic impairment appears to produce a clinically relevant effect elimination.
Volume of Distribution
Omadacycline has a mean Vd of 256 L after a single dose and a Vss of 190 L.
Clearance
Omadacycline has a mean systemic clearance of 11.24 L/h and a renal clearance of 2.4-3.3 L/h.
Omadacycline is not known to be metabolized in humans.
Omadacycline has a mean half life of elimination of 16.2 h.
Omadacycline binds to the primary tetracycline binding site on the bacterial 30s ribosomal subunit with high specificity. There it acts to block protein synthesis, disrupting many facets of cellular function and resulting in either cell death or stasis.


