



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
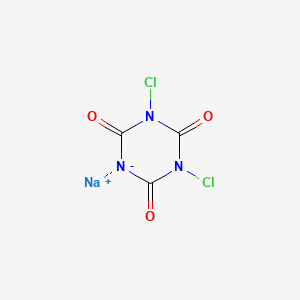

1. 1,3-dichloro-1,3,5-triazine-2,4,6(1h,3h,5h)-trione
2. Actichlor
3. Chlorcyanurate
4. Chlordesine
5. Chlordezine
6. Dichloroisocyanuric Acid
7. Dikon
8. Dikonit
9. Neoaquasept
10. Potassium Dichloro-s-triazinetrione
11. Presept
12. Sodium Dichloro-s-triazine-2,4,6(1h,3h,5h)-trione
13. Troclosene
14. Troclosene, Potassium Salt
15. Troclosene, Sodium Salt
16. Troclosene, Sodium Salt, Dihydrate
1. 2893-78-9
2. Dichloroisocyanuric Acid Sodium Salt
3. Troclosene Sodium
4. Sodium 3,5-dichloro-2,4,6-trioxo-1,3,5-triazinan-1-ide
5. Sodium Dichloro-s-triazinetrione
6. Dichloroisocyanuric Acid, Sodium Salt
7. 1260366-40-2
8. Sdic
9. Sodium Dichlorocyanurate
10. Sodium Troclosene
11. Ncgc00164091-01
12. Dsstox_cid_4994
13. Dsstox_rid_77616
14. Dsstox_gsid_24994
15. Actisept
16. Clearon
17. 07m9u9u0lk
18. Neochlor 55
19. Cas-2893-78-9
20. 1,3,5-triazine-2,4,6(1h,3h,5h)-trione, 1,3-dichloro-, Sodium Salt
21. Dichloro-s-triazinetrione Sodium Salt
22. Nadcc
23. Dichlorosan
24. 1,3,5-triazine-2,4,6(1h,3h,5h)-trione, 1,3-dichloro-, Sodium Salt (1:1)
25. Dichloroisocyanuric Acid Sodium
26. Schembl2485330
27. 3,5-dichloro-2-hydroxy-4,6-s-triazinedione Sodium Salt
28. Chembl3182790
29. Dtxsid3024994
30. Troclosene Sodium [hsdb]
31. Sodium Dichloroisocyanurate Powder
32. Sodium Dichloroisocyanurate, 96%
33. Troclosene Sodium [mart.]
34. Troclosene Sodium [who-dd]
35. Tox21_112083
36. Tox21_201601
37. Tox21_303262
38. Mfcd00006036
39. Akos024363213
40. Ncgc00257133-01
41. Ncgc00259150-01
42. Sodium Dichloroisocyanurate [jan]
43. Sodium Dichloroisocyanurate(sdic Or Nadcc)
44. D1003
45. Ft-0624714
46. J-017330
47. F0001-0906
48. Sodium3,5-dichloro-2,4,6-trioxo-1,3,5-triazinan-1-ide
49. Sodium;1,3-dichloro-1,3-diaza-5-azanidacyclohexane-2,4,6-trione
| Molecular Weight | 219.94 g/mol |
|---|---|
| Molecular Formula | C3Cl2N3NaO3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 218.9214405 g/mol |
| Monoisotopic Mass | 218.9214405 g/mol |
| Topological Polar Surface Area | 58.7 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 225 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
The microbiologic effectiveness of sodium dichloroisocyanurate (NaDCC) tablets used on a routine basis at the household level by a vulnerable population /was assessed/. In a 4-mo trial in Dhaka, Bangladesh, one half of the 100 participating households received NaDCC tablets and instructions on how to use the same; the other one half received a placebo and the same instructions. Monthly samples of stored drinking water from intervention households were significantly lower in thermotolerant coliforms (TTCs) than those of control households (geometric mean, 2.8 [95% CI: 2.2, 3.6] versus 604.1 [95% CI: 463.2, 787.9]; P < 0.0001). While 61.7% (116/188) of samples from the intervention households met World Health Organization (WHO) guidelines for 0 TTCs in drinking water, none of the 191 samples from control households met such a benchmark. Residual free chlorine in water samples suggested that householders consistently used the intervention, but 11.7% of samples exceeded the WHO guideline value of 5.0 mg/L, underscoring the need to ensure that tablet dose and vessel size are compatible.
PMID:17255252 Clasen T et al; Am J Trop Med Hyg 76 (1): 187-92 (2007).
3. 3 = moderately toxic: probable oral lethal dose (human) 0.5-5 g/kg, between 1 oz and 1 pint for 70 kg person (150 lb). /Trichloroisocyanuric acid/
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-77
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
Disinfectants
Substances used on inanimate objects that destroy harmful microorganisms or inhibit their activity. Disinfectants are classed as complete, destroying SPORES as well as vegetative forms of microorganisms, or incomplete, destroying only vegetative forms of the organisms. They are distinguished from ANTISEPTICS, which are local anti-infective agents used on humans and other animals. (From Hawley's Condensed Chemical Dictionary, 11th ed) (See all compounds classified as Disinfectants.)
Metabolism studies in both the rat and dog, following administration of (14)C-sodium isocyanurate by the oral and iv routes, demonstrated rapid absorption, distribution, and excretion of unmetabolized isocyanurate. ... At the 5 mg/kg dose, excretion was largely via the urine with about 5% in the feces. At the 500 mg/kg oral dose, 55-70% (rats) or 27-86% (dogs) was excreted in the feces and the remainder in the urine. /Sodium isocyanurate/
USEPA/Office of Pesticide Programs; Reregistration Eligibility Decision Document - Chlorinated Isocyanurates p.17 H-750BW (September, 1992). Available from, as of September 24, 2009: https://www.epa.gov/oppsrrd1/REDs/old_reds/chlorinated_isocyanurates.pdf
Both s-triazine-2,4,6(1H,3H,5H)-trione, 1,3,5-trichloro- and s-triazine-2,4,6(1H,3H,5H)-trione, 1,3-dichloro-, sodium salt are unstable in the body (particularly the stomach) because the available chlorine is rapidly reduced. Cyanuric acid (or its monosodium salt) is the degradation product from both products.
EPA/Office of Pollution Prevention and Toxics; High Production Volume Information System (HPVIS) on 1,3,5-Triazine-2,4,6(1H,3H,5H)-trione, 1,3,5-trichloro- (87-90-1). Available from, as of September 24, 2009: https://www.epa.gov/hpvis/index.html
Administration of (14C)sodium isocyanurate by the oral and iv routes demonstrated rapid absorption, distribution, and excretion of unmetabolized isocyanurate. The elimination half-life in rats was 32-43 min following 5 mg/kg iv or oral administration. The half-life was 122-148 min after oral dosing at 500 mg/kg.
USEPA/Office of Pesticide Programs; Reregistration Eligibility Decision Document - Chlorinated Isocyanurates p.17 H-750BW (September, 1992). Available from, as of September 24, 2009: https://www.epa.gov/oppsrrd1/REDs/old_reds/chlorinated_isocyanurates.pdf


