
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
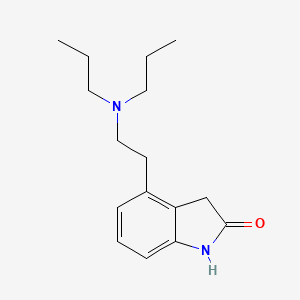

1. 4-(2-(di-n-propylamino)ethyl)-2(3h)-indolone
2. Requip
3. Ropinirol
4. Ropinirole Hydrochloride
5. Sk And F 101468
6. Sk And F-101,468
7. Skf 101468
1. 91374-21-9
2. Ropinirol
3. Ropinirolum
4. Ropinirolum [inn-latin]
5. Ropinirol [inn-spanish]
6. 4-(2-(dipropylamino)ethyl)indolin-2-one
7. Skf 101468
8. Sk&f 101468
9. 4-[2-(dipropylamino)ethyl]-1,3-dihydro-2h-indol-2-one
10. 4-[2-(dipropylamino)ethyl]-1,3-dihydroindol-2-one
11. Chembl589
12. Nsc-758917
13. Chebi:8888
14. 030pyr8953
15. Ncgc00015893-02
16. Dsstox_cid_25195
17. Dsstox_rid_80742
18. Dsstox_gsid_45195
19. Ropitor
20. Ropitor (tn)
21. Cas-91374-21-9
22. 4-(2-(dipropylamino)ethyl)-1,3-dihydro-2h-indol-2-one
23. Ropinirole [usan:inn:ban]
24. Unii-030pyr8953
25. Ropinirole [mi]
26. Ropinirole [inn]
27. Ropinirole (usan/inn)
28. Lopac-r-4152
29. Ropinirole [usan]
30. Ropinirole [vandf]
31. Ropinirole [who-dd]
32. Lopac0_001101
33. Schembl35212
34. Bidd:gt0826
35. Spectrum1505178
36. Gtpl7295
37. Zinc2041
38. Dtxsid8045195
39. Hsdb 8252
40. 2h-indol-2-one, 4-(2-(dipropylamino)ethyl)-1,3-dihydro-
41. Hms2093k04
42. Pharmakon1600-01505178
43. Bcp09383
44. Hy-b0623
45. Tox21_110256
46. Bdbm50020680
47. Nsc758917
48. Stl454344
49. Akos015843123
50. Tox21_110256_1
51. Ccg-205177
52. Db00268
53. Nsc 758917
54. Sb65618
55. Sdccgsbi-0051070.p002
56. Skf-101468
57. Sk&f-101468
58. 4-[2-(dipropylamino)ethyl]2-indolinone
59. Ncgc00015893-01
60. Ncgc00015893-03
61. Ncgc00015893-04
62. Ncgc00015893-06
63. Ncgc00094373-12
64. Ncgc00096064-01
65. Ncgc00096064-02
66. 4-[2-(dipropylamino)ethyl]-3h-indol-2-ol
67. Cs-0009561
68. Ft-0652500
69. C07564
70. D08489
71. Ab01563044_01
72. Ab01563044_02
73. 374r219
74. 4-(2-dipropylaminoethyl)-1,3-dihydroindol-2-one
75. L000520
76. Q420590
77. Sr-01000076215-3
78. Brd-k15933101-003-01-2
79. 4-[2-(dipropylamino)ethyl]-2,3-dihydro-1h-indol-2-one
80. 4-[2-(dipropylamino)ethyl]-1.3-dihydro-2h-indol-2-one
| Molecular Weight | 260.37 g/mol |
|---|---|
| Molecular Formula | C16H24N2O |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 7 |
| Exact Mass | 260.188863393 g/mol |
| Monoisotopic Mass | 260.188863393 g/mol |
| Topological Polar Surface Area | 32.3 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 287 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Requip |
| PubMed Health | Ropinirole (By mouth) |
| Drug Classes | Antiparkinsonian |
| Drug Label | REQUIP contains ropinirole, a non-ergoline dopamine agonist, as the hydrochloride salt. The chemical name of ropinirole hydrochloride is 4-[2-(dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-one and the empirical formula is C16H24N2OHCl. The molecular... |
| Active Ingredient | Ropinirole hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 4mg base; eq 5mg base; eq 0.5mg base; eq 2mg base; eq 0.25mg base; eq 1mg base; eq 3mg base |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 2 of 4 | |
|---|---|
| Drug Name | Requip xl |
| PubMed Health | Ropinirole (By mouth) |
| Drug Classes | Antiparkinsonian |
| Drug Label | REQUIPXL contains ropinirole, a nonergoline dopamine agonist as the hydrochloride salt. The chemical name of ropinirole hydrochloride is 4-[2-(dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-one and the empirical formula is C16H24N2OHCl. The mole... |
| Active Ingredient | Ropinirole hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | eq 4mg base; eq 2mg base; eq 6mg base; eq 12mg base; eq 8mg base |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 3 of 4 | |
|---|---|
| Drug Name | Requip |
| PubMed Health | Ropinirole (By mouth) |
| Drug Classes | Antiparkinsonian |
| Drug Label | REQUIP contains ropinirole, a non-ergoline dopamine agonist, as the hydrochloride salt. The chemical name of ropinirole hydrochloride is 4-[2-(dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-one and the empirical formula is C16H24N2OHCl. The molecular... |
| Active Ingredient | Ropinirole hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 4mg base; eq 5mg base; eq 0.5mg base; eq 2mg base; eq 0.25mg base; eq 1mg base; eq 3mg base |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 4 of 4 | |
|---|---|
| Drug Name | Requip xl |
| PubMed Health | Ropinirole (By mouth) |
| Drug Classes | Antiparkinsonian |
| Drug Label | REQUIPXL contains ropinirole, a nonergoline dopamine agonist as the hydrochloride salt. The chemical name of ropinirole hydrochloride is 4-[2-(dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-one and the empirical formula is C16H24N2OHCl. The mole... |
| Active Ingredient | Ropinirole hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | eq 4mg base; eq 2mg base; eq 6mg base; eq 12mg base; eq 8mg base |
| Market Status | Prescription |
| Company | Glaxosmithkline |
Antiparkinson Agents; Dopamine Agonists
National Library of Medicine's Medical Subject Headings. Ropinirole. Online file (MeSH, 2015). Available from, as of May 1, 2015: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health(NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Ropinirole is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of July 18, 2015: https://clinicaltrials.gov/search/intervention=Ropinirole
Requip is indicated for the treatment of Parkinson's disease. /Included in US product label/
NIH; DailyMed. Current Medication Information for Requip (Ropinirole Hydrochloride) Tablet, Film Coated (Updated: August 2014). Available from, as of June 10, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=de0bb94f-4fd8-4f27-5ba6-6f392dd5160f
Requip is indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS). /Included in US product label/
NIH; DailyMed. Current Medication Information for Requip (Ropinirole Hydrochloride) Tablet, Film Coated (Updated: August 2014). Available from, as of June 10, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=de0bb94f-4fd8-4f27-5ba6-6f392dd5160f
For more Therapeutic Uses (Complete) data for ROPINIROLE (7 total), please visit the HSDB record page.
Postmarketing reports indicate that patients may experience new or worsening mental status and behavioral changes, which may be severe, including psychotic-like behavior during treatment with Requip or after starting or increasing the dose of Requip. Other drugs prescribed to improve the symptoms of Parkinson's disease can have similar effects on thinking and behavior. This abnormal thinking and behavior can consist of one or more of a variety of manifestations including paranoid ideation, delusions, hallucinations, confusion, psychotic-like behavior, disorientation, aggressive behavior, agitation, and delirium.
NIH; DailyMed. Current Medication Information for Requip (Ropinirole Hydrochloride) Tablet, Film Coated (Updated: August 2014). Available from, as of June 10, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=de0bb94f-4fd8-4f27-5ba6-6f392dd5160f
Patients with a major psychotic disorder should ordinarily not be treated with Requip because of the risk of exacerbating the psychosis. In addition, certain medications used to treat psychosis may exacerbate the symptoms of Parkinson's disease and may decrease the effectiveness of Requip.
NIH; DailyMed. Current Medication Information for Requip (Ropinirole Hydrochloride) Tablet, Film Coated (Updated: August 2014). Available from, as of June 10, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=de0bb94f-4fd8-4f27-5ba6-6f392dd5160f
Safety and effectiveness in pediatric patients have not been established.
NIH; DailyMed. Current Medication Information for Requip (Ropinirole Hydrochloride) Tablet, Film Coated (Updated: August 2014). Available from, as of June 10, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=de0bb94f-4fd8-4f27-5ba6-6f392dd5160f
Ropinirole inhibits prolactin secretion in humans and could potentially inhibit lactation. Ropinirole has been detected in rat milk. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Requip is administered to a nursing woman.
NIH; DailyMed. Current Medication Information for Requip (Ropinirole Hydrochloride) Tablet, Film Coated (Updated: August 2014). Available from, as of June 10, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=de0bb94f-4fd8-4f27-5ba6-6f392dd5160f
For more Drug Warnings (Complete) data for ROPINIROLE (21 total), please visit the HSDB record page.
For the treatment of the signs and symptoms of Parkinson's disease and for the treatment of primary moderate-severe restless legs syndrome.
FDA Label
Induction of vomiting in dogs.
**Effects on Parkinson's and restless leg syndrome** This drug promotes the relief or improvement of symptoms of Parkinson's or restless leg syndrome by stimulatory actions on dopamine receptors, which regulate movement. **Effects on blood pressure** Clinical experience with dopamine agonists, including ropinirole, suggests an association with impaired abilities in regulating blood pressure with resulting orthostatic hypotension, especially with patients undergoing dose escalation. In some patients in clinical studies, blood pressure changes were associated with orthostatic symptoms, bradycardia, and, in one case in a healthy volunteer, transient sinus arrest accompanied by syncope. The mechanism of orthostatic hypotension caused by ropinirole is assumed to be due to a D2-mediated blunting of noradrenergic response to a standing position, followed by a decrease in peripheral vascular resistance. Nausea is also a frequent symptom which accompanies orthostatic signs and symptoms. **Effects on prolactin** At oral doses as low as 0.2 mg, ropinirole suppressed serum prolactin concentrations in healthy male volunteers. Ropinirole had no dose-related effect on ECG wave form and rhythm in young, healthy, male volunteers in the range of 0.01 to 2.5 mg. **Effects on QT interval** Ropinirole had no dose- or exposure-related effect on average QT intervals in healthy male and female volunteers at doses up to 4 mg/day. The effect of ropinirole on QTc intervals at higher exposures reached either due to drug interactions, hepatic dysfunction, or at higher doses has not been adequately evaluated.
Antiparkinson Agents
Agents used in the treatment of Parkinson's disease. The most commonly used drugs act on the dopaminergic system in the striatum and basal ganglia or are centrally acting muscarinic antagonists. (See all compounds classified as Antiparkinson Agents.)
Dopamine Agonists
Drugs that bind to and activate dopamine receptors. (See all compounds classified as Dopamine Agonists.)
QN04BC04
N04BC04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N04 - Anti-parkinson drugs
N04B - Dopaminergic agents
N04BC - Dopamine agonists
N04BC04 - Ropinirole
Absorption
Ropinirole is rapidly absorbed after oral administration, reaching peak concentration in approximately 1 to 2 hours,. Absolute bioavailability was 45% to 55%, suggesting approximately 50% hepatic first-pass effect. The bioavailability of ropinirole prolonged release compared to the immediate release tablets is about 100%. Ingestion of food does not affect the absorption of ropinirole, although its Tmax was increased by 2.5 hours and its Cmax was reduced by approximately 25% when the drug is taken with a high-fat meal.
Route of Elimination
The majority of the absorbed dose is cleared by the liver. In clinical trials, more than 88% of a radiolabeled dose was recovered in urine. Less than 10% of the administered dose is excreted as unchanged drug in urine. _N-despropyl ropinirole_ is the major metabolite found in the urine (40%), followed by the _carboxylic acid_ metabolite (10%), and the _glucuronide_ of the hydroxy metabolite (10%).
Volume of Distribution
Ropinirole is found to be widely distributed throughout the body, with an apparent volume of distribution of **7.5 L/kg**.
Clearance
The clearance of ropinirole after oral administration is 47 L/h.
The disposition and metabolic fate of ropinirole, a novel compound indicated for the symptomatic treatment of Parkinson's disease, was studied in the mouse, rat, cynomolgus monkey and man, following oral and intravenous administration of ropinirole hydrochloride. In all species, nearly all of the p.o. administered dose (94%) was rapidly absorbed from the gastrointestinal tract following administration of (14)C-ropinirole hydrochloride. In rat and monkey, the compound distributed rapidly beyond total body water and was shown to cross the blood-brain barrier. Blood clearance of the compound was high, approximately equal to one-half the hepatic blood flow in the monkey and similar to the hepatic blood flow in rat. Terminal phase elimination half-lives for the compound were relatively short (0.5 and 1.3 hr in rat and monkey respectively), although there was evidence of a second elimination phase in the monkey with an elimination half-life of approximately 5-11 hr. Plasma concentrations of ropinirole after the intravenous dose were not determined in the mouse and were below the lower limit of quantification in man (0.08 ng/mL) at the doses used in the studies described in this paper. In both animals and man, ropinirole was extensively metabolized. In the rat, the major metabolic pathway was via hydroxylation of the aromatic ring to form 7-hydroxy ropinirole. In mouse, monkey and man, the major pathway was via N-depropylation. The N-despropyl metabolite was metabolized further to form 7-hydroxy and carboxylic acid derivatives. Metabolites formed in all species were generally metabolized further by glucuronidation. 7-Hydroxy ropinirole is the only metabolite of ropinirole previously shown to possess significant dopamine agonist activity in vivo. In all species, the major route of excretion of ropinirole-related material after oral or intravenous administration of the compound was renal (60-90% of dose).
PMID:10219970 Ramji JV et al; Xenobiotica 29 (3): 311-25 (1999)
Ropinirole is a selective non-ergoline dopamine D2 receptor agonist indicated for use in treating Parkinson's disease. When taken as oral tablets, ropinirole is rapidly and almost completely absorbed, and it is extensively distributed from the vascular compartment. The bioavailability is approximately 50%. Ropinirole shows low plasma protein binding. The drug is inactivated by metabolism in the liver, and none of the major circulating metabolites have pharmacological activity. The principal metabolic enzyme is the cytochrome P450 (CYP) isoenzyme CYP1A2. Ropinirole shows approximately linear pharmacokinetics when given as single or repeated doses, and is eliminated with a half-life of approximately 6 hours. Population pharmacokinetics have demonstrated that gender, mild or moderate renal impairment, Parkinson's disease stage and concomitant illnesses or the use of several common concomitant medications have no effect on the pharmacokinetics of ropinirole. Clearance is slower for patients older than 65 years compared with those who are younger, and in women taking hormone replacement therapy compared with those who are not. The CYP1A2 inhibitor ciprofloxacin produced increases in the plasma concentrations of ropinirole when these 2 drugs were coadministered, but no interaction was seen with theophylline which, like ropinirole, is also a substrate for CYP1A2. There is no obvious plasma concentration-effect relationship for ropinirole.
PMID:11069211 Kaye CM, Nicholls B; Clin Pharmacokinet 39 (4): 243-54 (2000)
Ropinirole is widely distributed throughout the body, with an apparent volume of distribution of 7.5 L/kg. It is up to 40% bound to plasma proteins and has a blood-to-plasma ratio of 1:1.
NIH; DailyMed. Current Medication Information for Requip (Ropinirole Hydrochloride) Tablet, Film Coated (Updated: August 2014). Available from, as of June 10, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=de0bb94f-4fd8-4f27-5ba6-6f392dd5160f
Ropinirole is rapidly absorbed after oral administration, reaching peak concentration in approximately 1 to 2 hours. In clinical trials, more than 88% of a radiolabeled dose was recovered in urine and the absolute bioavailability was 45% to 55%, indicating approximately 50% first-pass effect. Relative bioavailability from a tablet compared with an oral solution is 85%. Food does not affect the extent of absorption of ropinirole, although its Tmax is increased by 2.5 hours and its Cmax is decreased by approximately 25% when the drug is taken with a high-fat meal.
NIH; DailyMed. Current Medication Information for Requip (Ropinirole Hydrochloride) Tablet, Film Coated (Updated: August 2014). Available from, as of June 10, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=de0bb94f-4fd8-4f27-5ba6-6f392dd5160f
For more Absorption, Distribution and Excretion (Complete) data for ROPINIROLE (6 total), please visit the HSDB record page.
Ropinirole is heavily metabolized by the liver. The most important metabolic pathways are N despropylation and hydroxylation to form the _N-despropyl_ metabolite and _hydroxy_ metabolites, both of which are inactive. The _N-despropyl_ metabolite is then converted to _carbamyl glucuronide_, carboxylic acid, and _N-despropyl hydroxy_ metabolites. Following this process, the _hydroxy_ metabolite of ropinirole is glucuronidated at a rapid rate. _In vitro_ studies show that the major cytochrome P450 enzyme involved in the metabolism of ropinirole is CYP1A2,.
Ropinirole is extensively metabolized by the liver. The N-despropyl metabolite is the major metabolite circulating in the plasma. Based on AUC data, the plasma levels of the metabolite were consistently higher than those of the parent drug suggesting a nonsaturable conversion of ropinirole to the N-despropyl metabolite. The affinity of the N-despropyl metabolite for human cloned D2 receptors is lower than the affinity of ropinirole. In addition the metabolite does not cross the blood-brain barrier; thus, it is unlikely to contribute to the therapeutic effects of ropinirole. The plasma concentrations of the hydroxylated metabolite are low and account for about 1-5% of the ropinirole concentrations. Although the hydroxylated metabolite was more active than ropinirole in in vitro D2 receptor binding studies, at therapeutic doses it is not expected to contribute to the activity of ropinirole.
Health Canada; Product Monograph for Requip (Ropinirole Hydrochloride) Tablets, Drug Identification Number (DIN): 02232565 p.28-9 (Date of Revision: May 7, 2014). Available from, as of June 10, 2015: https://webprod5.hc-sc.gc.ca/dpd-bdpp/start-debuter.do?lang=eng
The disposition and metabolic fate of ropinirole, a novel compound indicated for the symptomatic treatment of Parkinson's disease, was studied in the mouse, rat, cynomolgus monkey and man, following oral and intravenous administration of ropinirole hydrochloride. ... In the rat, the major metabolic pathway was via hydroxylation of the aromatic ring to form 7-hydroxy ropinirole. In mouse, monkey and man, the major pathway was via N-depropylation. The N-despropyl metabolite was metabolized further to form 7-hydroxy and carboxylic acid derivatives. Metabolites formed in all species were generally metabolized further by glucuronidation. 7-Hydroxy ropinirole is the only metabolite of ropinirole previously shown to possess significant dopamine agonist activity in vivo. ...
PMID:10219970 Ramji JV et al; Xenobiotica 29 (3): 311-25 (1999)
The dopamine receptor agonist ropinirole (SKF-101468) is used to treat Parkinson's disease. Ropinirole is metabolized by two routes to a series of different metabolites although the predominant pathway is species-dependent. It is unknown whether any of the metabolites contribute to its antiparkinsonian activity and whether D3 or D2 receptor agonist activity plays a preferential role. Therefore ropinirole and its primary metabolites, SKF-104557, SKF-97930 and SKF-96990, and the rat metabolite, SKF-89124 were tested in the 6-hydroxydopamine lesion model of Parkinson's disease. SKF-89124 and SKF-96990 were also assayed in radioligand binding and microphysiometer functional assays at cloned human dopamine D2 and D3. Ropinirole and SKF-89124 were equipotent in-vivo, and produced dose-related increases in circling at 0.05-0.8 mg kg(-1), s.c. (ropinirole) and 0.05-0.75 mg kg(-1), s.c. (SKF-89124). Neither SKF-96990 or SKF-97930, at doses up to 15 mg kg(-1), increased the circling rate. Some circling was observed with 15 mg kg(-1) SKF-104557 but the response was less than half that produced by ropinirole (0.8 mgkg(-1)). SKF-104557 was 150-fold less potent than ropinirole. SKF-89124 possessed-30-fold higher affinity for D3 over D2 receptors in radioligand binding studies, but was not selective in the functional microphysiometer assay. SKF-96990 was 10-fold selective for D3 over D2 receptors in the radioligand binding assay. Ropinirole and SKF-104557 are 20-fold selective for D3 over D2 receptors in radioligand binding assays whereas in microphysiometry, selectivity is 10-fold. SKF-97930 is inactive in radioligand binding and microphysiometer assays. Primary metabolites of ropinirole did not contribute significantly to its activity in this model of Parkinson's disease. The lack of dopamine D3/D2 receptor selectivity for ropinirole rules out the possibility of attributing the degree of either D2 or D3 receptor activity to the behavioural efficacy of ropinirole.
PMID:1104589 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC236065 Reavill C et al; J Pharm Pharmacol 52 (9): 1129-35 (2000)
The in vitro metabolism of ropinirole was investigated with the aim of identifying the cytochrome P450 enzymes responsible for its biotransformation. The pathways of metabolism after incubation of ropinirole with human liver microsomes were N-despropylation and hydroxylation. Enzyme kinetics demonstrated the involvement of at least two enzymes contributing to each pathway. A high affinity component with a K(M) of 5-87 uM and a low affinity component with a K(M) of approximately two orders of magnitude greater were evident. The high affinity component could be abolished by pre-incubation of the microsomes with furafylline. Additionally, incubation of ropinirole with microsomes derived from CYP1A2 transfected cells readily produced the N-despropyl and hydroxy metabolites. Some inhibition of ropinirole metabolism was also observed with ketoconazole, indicating a minor contribution by CYP3A. Multivariate correlation data were consistent with the involvement of the cytochrome P450 enzymes 1A2 and 3A in the metabolism of ropinirole. Thus, it could be concluded that the major P450 enzyme responsible for ropinirole metabolism at lower (clinically relevant) concentrations is CYP1A2 with a contribution from CYP3A, particularly at higher concentrations.
PMID:9224778 Bloomer JC et al; Drug Metab Dispos 25 (7): 840-4 (1997)
Ropinirole has known human metabolites that include 4-(2-(Propylamino)ethyl)indolin-2-one and 4-[2-(Dipropylamino)ethyl]-7-hydroxy-1,3-dihydro-2h-indol-2-one.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Approximately 6 hours,.
The terminal elimination half-life is approximately 6 hr (range 2-27 hr) ... .
Health Canada; Product Monograph for Requip (Ropinirole Hydrochloride) Tablets, Drug Identification Number (DIN): 02232565 p.27 (Date of Revision: May 7, 2014). Available from, as of June 10, 2015: https://webprod5.hc-sc.gc.ca/dpd-bdpp/start-debuter.do?lang=eng
The disposition and metabolic fate of ropinirole, a novel compound indicated for the symptomatic treatment of Parkinson's disease, was studied in the mouse, rat, cynomolgus monkey and man, following oral and intravenous administration of ropinirole hydrochloride. ... Terminal phase elimination half-lives for the compound were relatively short (0.5 and 1.3 hr in rat and monkey respectively), although there was evidence of a second elimination phase in the monkey with an elimination half-life of approximately 5-11 hr. ...
PMID:10219970 Ramji JV et al; Xenobiotica 29 (3): 311-25 (1999)
Ropinirole is a non-ergoline dopamine agonist. Ropinirole has the highest affinity at the D3 receptors, which are concentrated in the limbic areas of the brain and may be responsible for some of the neuropsychiatric effects. The exact mechanism of action of ropinirole as a treatment for Parkinsons disease is unknown, however, it is believed to be related to its ability to selectively stimulate dopamine D2 receptors within the caudate-putamen system in the brain. This system affects body movement. Negligible affinity is seen for ropinirole at 2 adrenoreceptors in the periphery and 5HT-1 receptor. Ropinirole has no affinity at the D1-like receptors, benzodiazepine or GABA receptors. The precise mechanism of action of ropinirole as a treatment for Restless Legs Syndrome is unknown, however, it is believed to be related to its ability to stimulate dopamine receptors.
The present study determined its affinity and agonist efficacy at recombinant human (h) dopamine hD2, hD3 and hD4 and serotonin (5-HT) h5-HT1A, h5-HT1B and h5-HT1D receptors. Roxindole exhibited high affinity at hD3 as well as at hD2 (short isoform) and hD4 (4-repeat isoform) receptors (pKi values 8.93, 8.55 and 8.23, respectively). Further, it displayed high affinity at hS-HT1A receptors (pKi = 9.42) but modest affinity at 5-HT1B and 5-HT1D receptors (pKi values 6.00 and 7.05, respectively). In [35S]GTPgammaS binding experiments, roxindole was >20-fold more potent in stimulating [35S]GTPgammaS binding at hD3 than at hD2 or hD4 receptors (pEC50 = 9.23 vs. 7.88 and 7.69). However, whereas roxindole exhibited partial agonist activity at hD3 and hD4 sites (Emax = 30.0% and 35.1%, respectively, relative to dopamine = 100%), it only weakly activated hD2 receptors (Emax = 10.5%). Roxindole potently blocked dopamine-stimulated [35S]GTPgammaS binding at hD2 receptors (pkappaB = 9.05). In comparison, the dopamine receptor agonist, (-)quinpirole, acted as a partial agonist at hD3 and hD4 sites (Emax = 67.4% and 66.3%, respectively) but surpassed the efficacy of dopamine at hD2 receptors (Emax = 132%). At h5-HT1A receptors, roxindole behaved as a high affinity (pKi = 9.42) partial agonist (Emax = 59.6%, relative to 5-HT = 100%), whereas (-)quinpirole had negligible activity. The selective 5-HT1A antagonist, WAY 100,635, blocked roxindole (100 nM)-stimulated [35S]GTPgammaS binding at h5-HT1A receptors in a concentration-dependent manner (pkappaB = 9.28). Roxindole only weakly stimulated [35S]GTPgammaS binding at 5-HT1B and 5-HT1D receptors (Emax = 27.1% and 13.7%). The present data suggest that roxindole activates mainly D3 vs. D2 or D4 receptors and 5-HT1A vs. 5-HT1B or 5-HT1D receptors. Activation of D3 and/or 5-HT1A receptors may thus contribute to its potential antidepressant properties.
PMID:10431754 Newman-Tancredi A et al; Naunyn Schmiedebergs Arch Pharmacol 359 (6): 447-53 (1999)
The aim of the present study was to characterize functional responses to ropinirole, its major metabolites in man (SKF-104557 (4-[2-(propylamino)ethyl]-2-(3H) indolone), SKF-97930 (4-carboxy-2-(3H) indolone)) and other dopamine receptor agonists at human dopamine D2(long) (hD2), D3 (hD3) and D4.4 (hD4) receptors separately expressed in Chinese hamster ovary cells using microphysiometry. 2. All the receptor agonists tested (ropinirole, SKF-104557, SKF-97930, bromocriptine, lisuride, pergolide, pramipexole, talipexole, dopamine) increased extracellular acidification rate in Chinese hamster ovary clones expressing the human D2, D3 or D4 receptor. The pEC50s of ropinirole at hD2, hD3 and hD4 receptors were 7.4, 8.4 and 6.8, respectively. Ropinirole is therefore at least 10 fold selective for the human dopamine D3 receptor over the other D2 receptor family members. 3. At the hD2 and hD3 dopamine receptors all the compounds tested were full agonists as compared to quinpirole. Talipexole and the ropinirole metabolite, SKF-104557, were partial agonists at the hD4 receptor. 4. Bromocriptine and lisuride had a slow onset of agonist action which precluded determination of EC50s. 5. The rank order of agonist potencies was dissimilar to the rank order of radioligand binding affinities at each of the dopamine receptor subtypes. Functional selectivities of the dopamine receptor agonists, as measured in the microphysiometer, were less than radioligand binding selectivities. 6. The results show that ropinirole is a full agonist at human D2, D3 and D4 dopamine receptors. SKF-104557 the major human metabolite of ropinirole, had similar radioligand binding affinities to, but lower functional potencies than, the parent compound.
PMID:10455328 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1566138 Coldwell MC et al; Br J Pharmacol 127 (7): 1696-702 (1999)
Ropinirole, which is a non-ergot dopamine agonist derivative, exerts therapeutic benefits in Parkinson's disease (PD). Based on recent studies implicating dopamine receptors 2 and 3 (D2R and D3R) as possible targets of ropinirole, we over-expressed these dopamine receptor genes in the dopamine-denervated striatum of rodents to reveal whether their over-expression modulated ropinirole activity. Adult Sprague-Dawley rats initially received unilateral 6-hydroxydopamine lesion of the medial forebrain bundle. At 1 month after surgery, successfully lesioned animals (3 or less forelimb akinesia score, and 8 or more apomorphine-induced rotations/min over 1 hr) were randomly assigned to intrastriatal injection (ipsilateral to the lesion) of blank lentiviral vector, D2R, D3R or both genes. At about 5 months post-lesion, ropinirole (0.2 mg/kg, i.p.) was administered daily for 9 consecutive days. The subtherapeutic dose of ropinirole improved the use of previously akinetic forelimb and produced robust circling behavior in lesioned animals with striatal over-expression of both D2R and D3R compared to lesioned animals that received blank vector. In contrast, the subtherapeutic dose of ropinirole generated only modest motor effects in lesioned animals with sole over-expression of D2R or D3R. Western immunoblot and autoradiographic assays showed enhanced D2R and D3R protein levels coupled with normalized D2R and D3R binding in the ventral striatum of lesioned animals with lentiviral over-expression of both D2R and D3R relative to vehicle-treated lesioned animals. Immunohistochemical analyses showed that D2R and D3R GFP fluorescent cells colocalized with enkephalin and substance P immunoreactive medium spiny neurons. These data support the use of the subtherapeutic dose of ropinirole in a chronic model of PD.
PMID:17573046 Matsukawa N et al; Brain Res 1160: 113-23 (2007)
Ropinirole hydrochloride, a dipropylaminoethyl indolone derivative, is a nonergot-derivative dopamine receptor agonist. In in vitro binding studies, ropinirole demonstrated high binding specificity for and intrinsic activity at dopamine D2 receptors compared with other dopamine receptor agonists (e.g., bromocriptine, pergolide), having a higher affinity for the D3 subtype than for the D2 or D4 subtypes. Ropinirole binds with moderate affinity to opiate receptors but has little or no affinity for alpha1-, alpha2-, or beta-adrenergic; dopamine D1; benzodiazepine; gamma-aminobutyric acid (GABA); serotonin type 1 (5-hydroxytryptamine (5-HT1)); serotonin type 2 (5-HT2); or muscarinic receptors.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2665

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
54
PharmaCompass offers a list of Ropinirole API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ropinirole manufacturer or Ropinirole supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ropinirole manufacturer or Ropinirole supplier.
PharmaCompass also assists you with knowing the Ropinirole API Price utilized in the formulation of products. Ropinirole API Price is not always fixed or binding as the Ropinirole Price is obtained through a variety of data sources. The Ropinirole Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A 030PYR8953 manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of 030PYR8953, including repackagers and relabelers. The FDA regulates 030PYR8953 manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. 030PYR8953 API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of 030PYR8953 manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A 030PYR8953 supplier is an individual or a company that provides 030PYR8953 active pharmaceutical ingredient (API) or 030PYR8953 finished formulations upon request. The 030PYR8953 suppliers may include 030PYR8953 API manufacturers, exporters, distributors and traders.
click here to find a list of 030PYR8953 suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A 030PYR8953 DMF (Drug Master File) is a document detailing the whole manufacturing process of 030PYR8953 active pharmaceutical ingredient (API) in detail. Different forms of 030PYR8953 DMFs exist exist since differing nations have different regulations, such as 030PYR8953 USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A 030PYR8953 DMF submitted to regulatory agencies in the US is known as a USDMF. 030PYR8953 USDMF includes data on 030PYR8953's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The 030PYR8953 USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of 030PYR8953 suppliers with USDMF on PharmaCompass.
A 030PYR8953 written confirmation (030PYR8953 WC) is an official document issued by a regulatory agency to a 030PYR8953 manufacturer, verifying that the manufacturing facility of a 030PYR8953 active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting 030PYR8953 APIs or 030PYR8953 finished pharmaceutical products to another nation, regulatory agencies frequently require a 030PYR8953 WC (written confirmation) as part of the regulatory process.
click here to find a list of 030PYR8953 suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing 030PYR8953 as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for 030PYR8953 API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture 030PYR8953 as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain 030PYR8953 and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a 030PYR8953 NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of 030PYR8953 suppliers with NDC on PharmaCompass.
030PYR8953 Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of 030PYR8953 GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right 030PYR8953 GMP manufacturer or 030PYR8953 GMP API supplier for your needs.
A 030PYR8953 CoA (Certificate of Analysis) is a formal document that attests to 030PYR8953's compliance with 030PYR8953 specifications and serves as a tool for batch-level quality control.
030PYR8953 CoA mostly includes findings from lab analyses of a specific batch. For each 030PYR8953 CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
030PYR8953 may be tested according to a variety of international standards, such as European Pharmacopoeia (030PYR8953 EP), 030PYR8953 JP (Japanese Pharmacopeia) and the US Pharmacopoeia (030PYR8953 USP).
