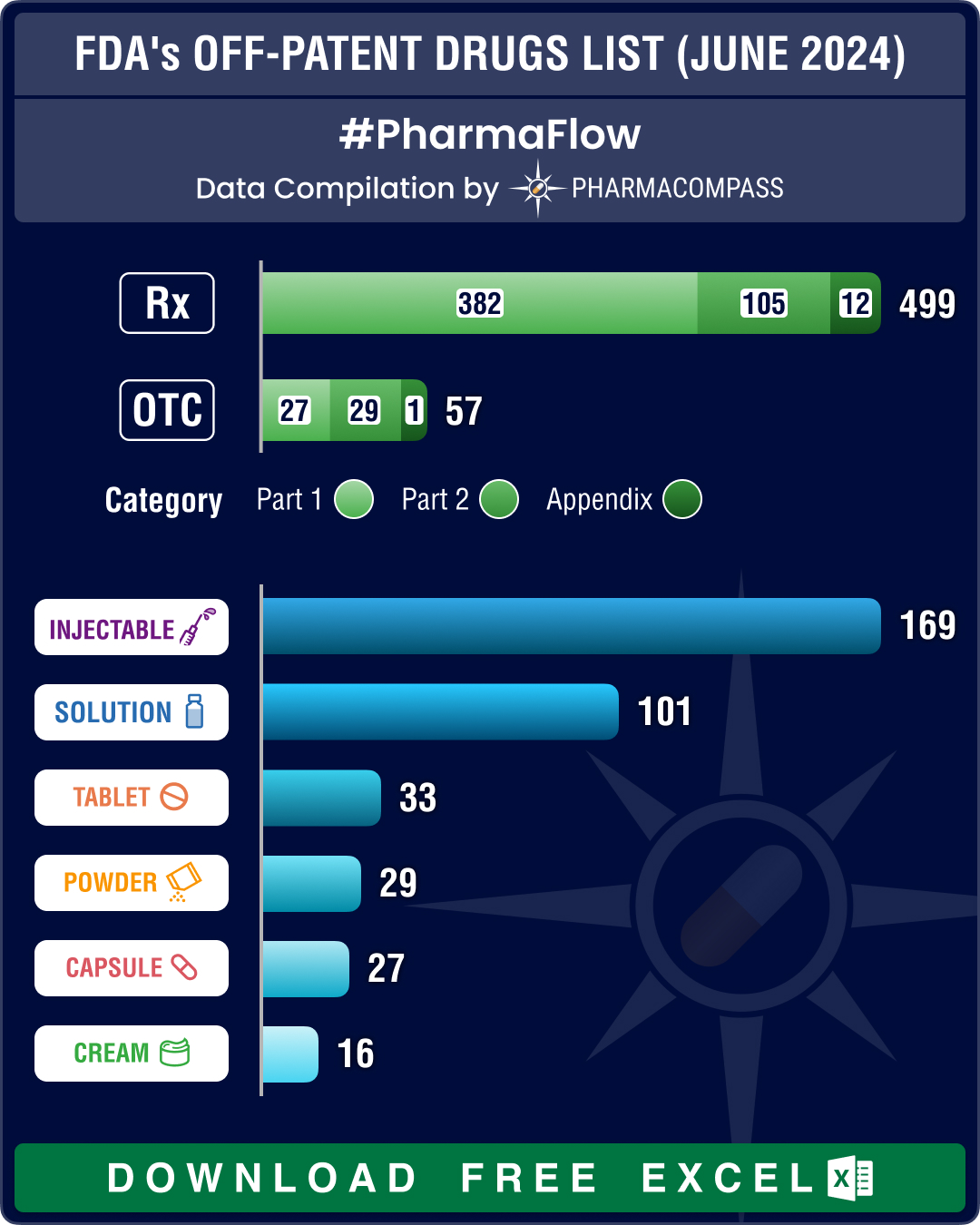
FDA’s December 2025 OPOE list features 784 prescription drugs, 73 OTC drugs
This
week, PharmaCompass brings you key highlights of the US Food and Drug Administration’s D
DMF filings rise 4.5% in Q3 2025; China holds lead, India records 20% growth in submissions
The
third quarter (Q3) of 2025 witnessed a steady rise in Drug Master File (DMF) submissions to the
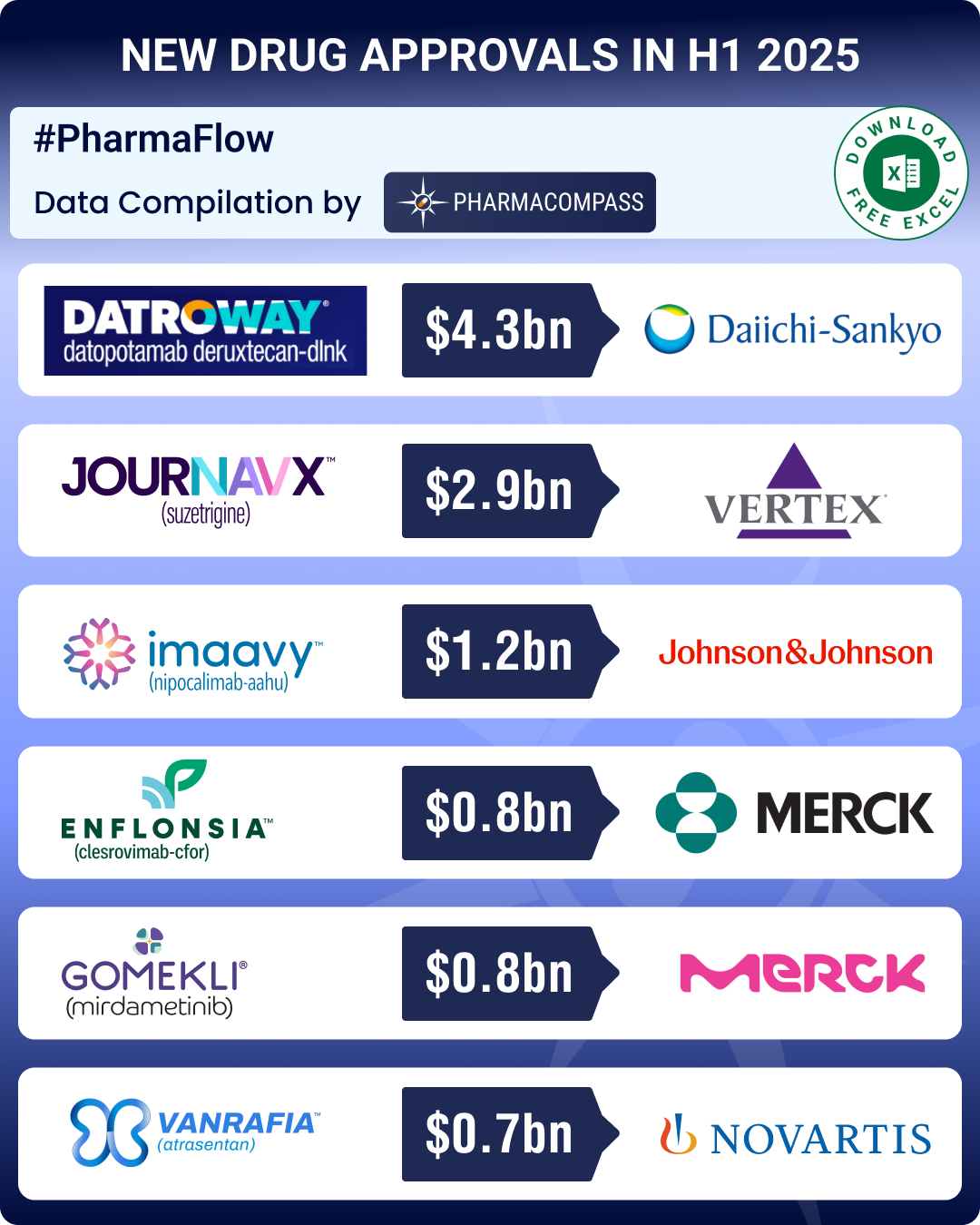
FDA approvals drop 24% in H1 2025; GSK’s UTI med, Vertex’s non-opioid painkiller lead pack of first-in-class meds
It has been a turbulent year for the US
Food and Drug Administration (FDA), marked by reduction
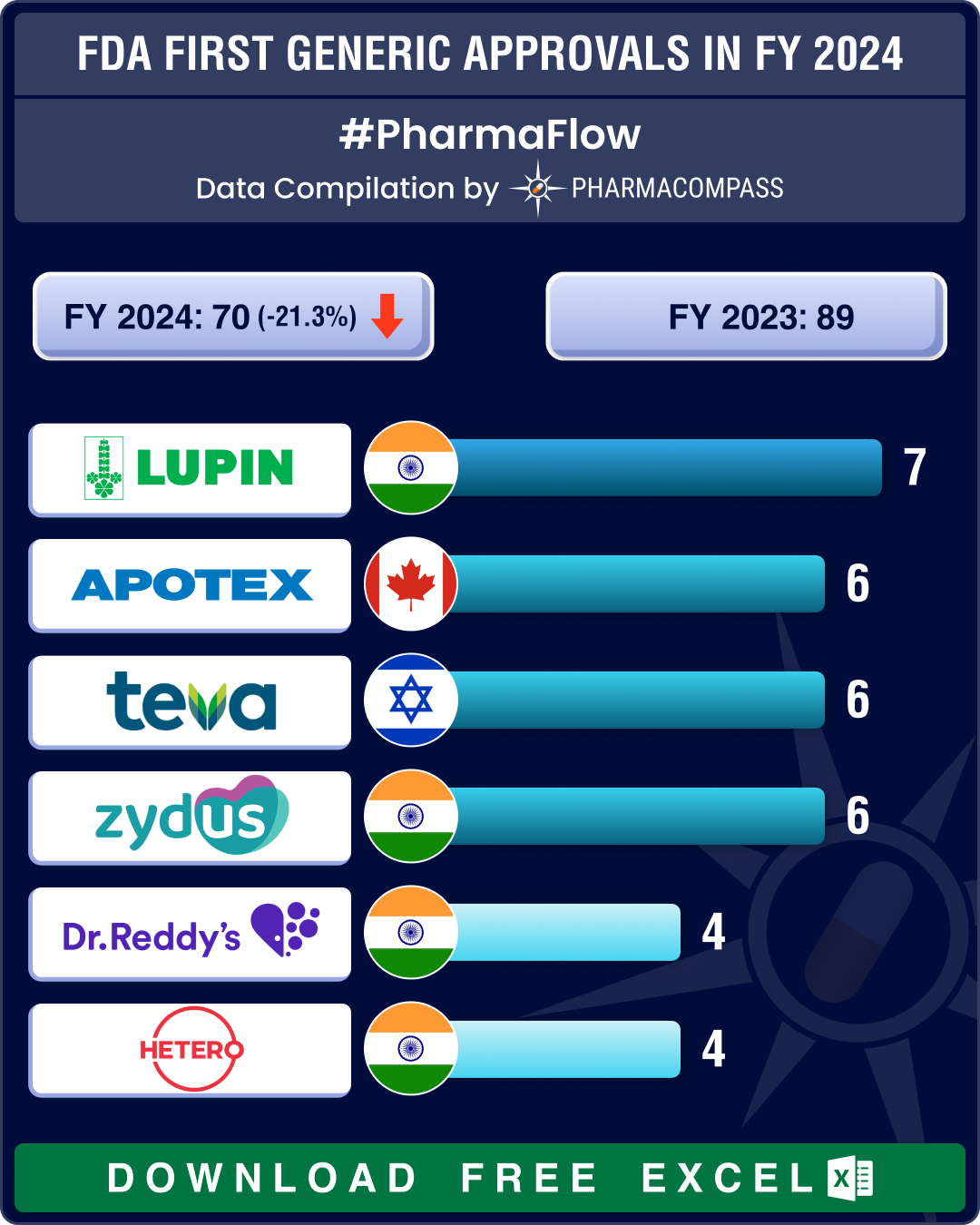
FDA’s first generic approvals slump 21% in 2024; Novartis’ top seller Entresto, cancer blockbuster Tasigna lead 2024 patent cliff
A watershed moment in the journey of a drug is when it transitions from being a patented, high‐


 Market Place
Market Place Sourcing Support
Sourcing Support