

X


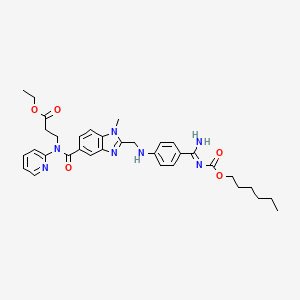

1. N-[2-[4-[n-(hexyloxycarbonyl)amidino]phenylaminomethyl]-1-methyl-1h-benzimidazol-5-ylcarbonyl]-n-(2-pyridyl)-beta-alanine Ethyl Ester
2. Rabigatran Etexilate
3. Bibr1048
4. Bcpp000269
5. Bibr-1048 - Dabigatran Etexilate
6. Akos015900928
7. Am81239
8. Bcp9000578
9. Bs-1001
10. Ncgc00390544-02
11. Ab01563294_01
12. 915b069
13. Q-102504
14. Hexyl2-(4-(((5-((3-ethoxy-3-oxopropyl)(pyridin-2-yl)carbamoyl)-1-methyl-1h-benzo[d]imidazol-2-yl)methyl)amino)benzylidene)hydrazinecarboxylate
| Molecular Weight | 627.7 g/mol |
|---|---|
| Molecular Formula | C34H41N7O5 |
| XLogP3 | 5.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 17 |
| Exact Mass | 627.31691743 g/mol |
| Monoisotopic Mass | 627.31691743 g/mol |
| Topological Polar Surface Area | 154 Ų |
| Heavy Atom Count | 46 |
| Formal Charge | 0 |
| Complexity | 1000 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |







