




1. 241479-73-2
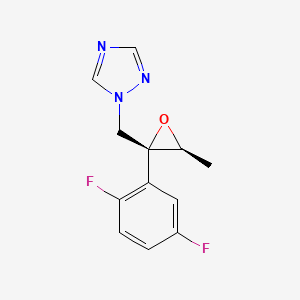
2. (2r,3s)-2-(2,5-difluorophenyl)-3-methyl-2-[(1h-1,2,4-triazol-1-yl)-methyl]-oxirane
3. 1-[[(2r,3s)-2-(2,5-difluorophenyl)-3-methyloxiran-2-yl]methyl]-1,2,4-triazole
4. 1-[[(2r,3s)-2-(2,5-difluorophenyl)-3-methyloxiranyl]methyl]-1h-1,2,4-triazole
5. Schembl1828222
6. Zinc34279570
7. Bs-51355
8. A854828
9. A1-03608
10. 1-[[(2r,3s)-2-(2,5-difluorophenyl)-3-methyloxiranyl]methyl]-1h-1,2,4-
11. (2r,3s)-2-(2,5-difluorophenyl)-3-methyl-2-[(1h-1,2,4-triazol-1-yl)methyl]oxirane
12. (2r,3s)-2-(2,5-difluorophenyl)-3-methyl-2-[(1h-1,2,4-triazole-1-yl)methyl]oxirane
| Molecular Weight | 251.23 g/mol |
|---|---|
| Molecular Formula | C12H11F2N3O |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 251.08701831 g/mol |
| Monoisotopic Mass | 251.08701831 g/mol |
| Topological Polar Surface Area | 43.2 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 320 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |



