




1. 14489-75-9
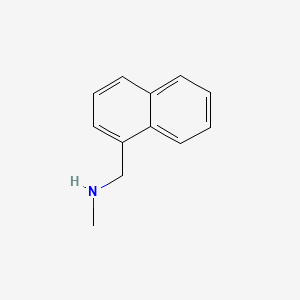
2. N-methyl-1-naphthylmethylamine
3. N-methyl-1-(naphthalen-1-yl)methanamine
4. N-methyl-1-naphthalenemethylamine
5. N-methylnaphthalene-1-methylamine
6. 1-methyl-aminomethyl-naphthalene
7. 1-naphthalenemethanamine, N-methyl-
8. N-methyl-1-naphthalen-1-ylmethanamine
9. 1-(methylaminomethyl)naphthalene
10. N-methyl-1-naphthalenemethanamine
11. Methyl[(naphthalen-1-yl)methyl]amine
12. Dwx2r0ps9p
13. Methyl-1-naphthalenemethylamine
14. N-methyl-1-naphthalen-1-yl-methanamine
15. Mfcd00144934
16. Nsc-129392
17. N-methyl-1-naphthalenemethyl Amine
18. Unii-dwx2r0ps9p
19. N-methylnaphthalenemethylamine
20. Einecs 238-497-3
21. Terbinafine Ep Impurity A
22. Ec 238-497-3
23. N-methyl-naphthylmethylamine
24. Methyl(1-naphthylmethyl)amine
25. Schembl681401
26. Methyl-(1-naphthylmethyl)amine
27. N-methyl-1-naphthylmethyl Amine
28. N-methyl(1-naphthyl)methanamine
29. Dtxsid00162834
30. N-methyl-(1-naphthylmethyl)amine
31. Methyl-naphthalen-1-ylmethyl-amine
32. N-methyl-1-naphthalene Methanamine
33. N-methyl Naphthylmethylamine
34. N-methyl(1-naphthyl)methanamine #
35. Bcp30014
36. Zinc1671536
37. N-methyl-n-(1-naphthylmethyl)amine
38. Nsc129392
39. Stk728698
40. Akos000129852
41. Ab03923
42. Ac-2115
43. Cs-w007897
44. Fs-1321
45. Nsc 129392
46. N-methyl-1-naphthalenmethylamine
47. Sy017660
48. Db-042756
49. Am20050281
50. Ft-0637091
51. Ft-0672629
52. M0983
53. En300-35038
54. 89m759
55. F16464
56. Terbinafine Related Compound A Free Base
57. Ab00633513-02
58. N-methyl-c-(naphthalen-1-yl)methanamine
59. N-methyl-n-((naphthalen-1-yl)methyl)amine
60. W-108146
61. Q27276647
62. Terbinafine Hydrochloride Impurity A [ep Impurity]
63. Terbinafine Hydrochloride Impurity, N-methyl-c-(naphthalen-1-yl)methanamine- [usp Impurity]
| Molecular Weight | 171.24 g/mol |
|---|---|
| Molecular Formula | C12H13N |
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 12 |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 155 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
 IKF/Pharmasynthese have been with fine chemicals market and APIs performance for more than 40 years.
IKF/Pharmasynthese have been with fine chemicals market and APIs performance for more than 40 years. 




