



1. 74163-81-8
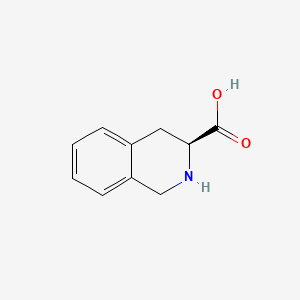
2. L-1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid
3. (3s)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid
4. L-porretine
5. H-tic-oh
6. (s)-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic Acid
7. (s)-(-)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid
8. (3s)-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic Acid
9. S-(-)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid
10. 3-isoquinolinecarboxylic Acid, 1,2,3,4-tetrahydro-, (3s)-
11. 737g46u1np
12. 1,2,3,4-tetrahydroisoquinoline-3(s)-carboxylic Acid
13. (-)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid
14. (s)-1,2,3,4-tetrahydro-3-isoquinoline-carboxylic Acid
15. 1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid, (s)-
16. (s)-(-)-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic Acid
17. Mfcd00144533
18. L-tic
19. Unii-737g46u1np
20. H-l-tic-oh
21. (r)-(+)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid
22. Schembl288272
23. S-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic Acid
24. Chembl447576
25. Act01929
26. Cs-d1187
27. Str03867
28. Zinc3881715
29. Akos016842273
30. Ts-02454
31. A9522
32. Am20060719
33. J1119
34. T1515
35. 163t818
36. J-650310
37. L-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic Acid
38. Q-103215
39. 1,2,3,4-tetrahydro-3(s)-isoquinolinecarboxylic Acid
40. 1,2,3,4-tetrahydroisoquinoline-(3s)-carboxylic Acid
41. Q27266148
42. S-1,2,3,4-tetrahydro-3-isoquinoline Carboxylic Acid
43. (3s)-1,2,3,4-tetrahydro-3-isoquinolinecarbocylic Acid
44. (3s)-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic Acid
45. L-(3s)1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid
46. Quinapril Hydrochloride Impurity A [ep Impurity]
47. Z1696862396
48. (s)-(-)-1,2,3,4-tetrahydro-3-isoquindinecarboxylic Acid
49. (s)-(-)-1,2,3,4-tetrahydro-3-isoquinoline-carboxylic Acid
50. (s)-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic Acid, 97%
51. 1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid, (-)-
52. 1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid, (3s)-
| Molecular Weight | 177.20 g/mol |
|---|---|
| Molecular Formula | C10H11NO2 |
| XLogP3 | -1.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 177.078978594 g/mol |
| Monoisotopic Mass | 177.078978594 g/mol |
| Topological Polar Surface Area | 49.3 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 205 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Note: None of the products will be supplied to countries in which this could be in conflict with existing patents. Further, any products under patent will be offered for R&D purposes only. However, the final responsibility lies with the buyer.




