

X


1. 855778-84-6
2. Schembl2209444
3. Bapaubkdzoewjl-uhfffaoysa-n
4. E72264
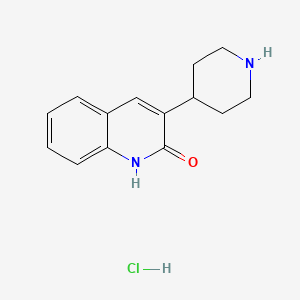
5. 3-(piperidin-4-yl)quinolin-2(1h)-one-hydrochloride
6. 3-(4-piperidinyl)-2(1h)-quinolinone Hydrochloride
| Molecular Weight | 264.75 g/mol |
|---|---|
| Molecular Formula | C14H17ClN2O |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 41.1 |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 331 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
 Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.
Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs. 



