



1. 69759-61-1
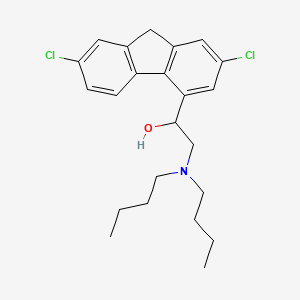
2. 2-(dibutylamino)-1-(2,7-dichloro-9h-fluoren-4-yl)ethanol
3. 2,7-dichloro-alpha-[(dibutylamino)-methyl]-9h-fluorene-4-methanol
4. Schembl5808111
5. Chembl3230061
6. Dtxsid70330760
7. Mfcd12923306
8. Akos015899558
9. Sb66888
10. As-10058
11. Db-024460
12. A9224
13. Cs-0152113
14. Ft-0660547
15. D95749
16. 221d071
17. J-507537
18. 2-(di-n-butylamino)-1-[2,7-dichloro-9h-fluoren-4-yl]ethanol
19. 2-(dibutylamino)-1-(2,7-dichloro-9h-fluoren-4-yl)ethanol Hcl
20. 2-(dibutylamino)-1-(2,7-dichloro-9h-fluoren-4-yl)ethan-1-ol
| Molecular Weight | 406.4 g/mol |
|---|---|
| Molecular Formula | C23H29Cl2NO |
| XLogP3 | 6.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 9 |
| Exact Mass | 405.1626199 g/mol |
| Monoisotopic Mass | 405.1626199 g/mol |
| Topological Polar Surface Area | 23.5 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 440 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |




