

X



1. 521284-19-5
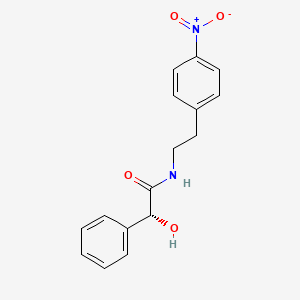
2. (2r)-2-hydroxy-n-[2-(4-nitrophenyl)ethyl]-2-phenylacetamide
3. (r)-n-(4-nitrophenethyl)-2-hydroxy-2-phenylacetamide
4. (alphar)-alpha-hydroxy-n-[2-(4-nitrophenyl)ethyl]benzeneacetamide
5. Schembl1405355
6. Mfcd22200231
7. Zinc73032732
8. Akos024464728
9. Ds-7812
10. Ac-26408
11. Cs-0149883
12. (r)-n-(4-nitrophenethyl)-2-phenyl-2-hydroxyacetamide
13. (r)-2-hydroxy-n-[2-(4-nitro-phenyl)ethyl]-2-phenylacetamide
14. (r)-2-hydroxy-n-[2-(4-nitrophenyl)ethyl]-2-phenylacetamide
| Molecular Weight | 300.31 g/mol |
|---|---|
| Molecular Formula | C16H16N2O4 |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Exact Mass | 300.11100700 g/mol |
| Monoisotopic Mass | 300.11100700 g/mol |
| Topological Polar Surface Area | 95.2 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 369 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |







