
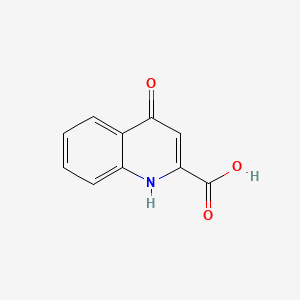

1. Acid, Kynurenic
2. Kynurenate
1. 4-hydroxyquinoline-2-carboxylic Acid
2. 492-27-3
3. 13593-94-7
4. Transtorine
5. Kynurenate
6. 4-oxo-1,4-dihydroquinoline-2-carboxylic Acid
7. Quinurenic Acid
8. 4-hydroxyquinaldic Acid
9. 4-hydroxy-2-quinolincarboxylic Acid
10. Kinurenic Acid
11. Kynuronic Acid
12. 1,4-dihydro-4-oxoquinoline-2-carboxylic Acid
13. 2-quinolinecarboxylic Acid, 4-hydroxy-
14. 4-hydroxy-2-quinolinecarboxylic Acid
15. 4-hydroxyquinaldinic Acid
16. 4-oxo-1h-quinoline-2-carboxylic Acid
17. Quinaldic Acid, 4-hydroxy-
18. Kyna
19. Nsc 58973
20. Kynurenic-acid
21. Chebi:18344
22. Nsc58973
23. Nsc-58973
24. 2-carboxy-4-hydroxyquinoline
25. Chembl299155
26. H030s2s85j
27. 4-oxo-1,4-dihydro-quinoline-2-carboxylic Acid
28. 1,4-dihydro-4-oxoquinoline-2-carboxylicacid
29. 4-hydroxyquinoline-2-carboxylate
30. Ccris 4428
31. Sr-01000075455
32. Einecs 207-751-5
33. Kynurensaeure
34. Unii-h030s2s85j
35. 4-hydroxyquinaldate
36. Kya
37. Mfcd00006753
38. 4-hydroxy-quinaldate
39. 4-hydroxyquinaldinate
40. Spectrum_001116
41. Tocris-0223
42. 4-hydroxy-quinaldic Acid
43. Spectrum2_001342
44. Spectrum3_001390
45. Spectrum4_000814
46. Spectrum5_001318
47. Lopac-k-3375
48. Quinurenic Acid
49. Kynurenate
50. Biomol-nt_000229
51. Bmse000410
52. Kynurenic Acid, >=98%
53. Kynurenic Acid [mi]
54. Lopac0_000716
55. Oprea1_032085
56. Schembl22979
57. Bspbio_002980
58. Kbiogr_001327
59. Kbioss_001596
60. Mls002172436
61. Divk1c_000309
62. Spectrum1500688
63. Spbio_001523
64. Bpbio1_001350
65. Gtpl2918
66. Dtxsid8075417
67. 4-hydroxy-2-chinolincarbonsaeure
68. Bdbm81975
69. Hczhheifkropdy-uhfffaoysa-
70. Hms500p11
71. Kbio1_000309
72. Kbio2_001596
73. Kbio2_004164
74. Kbio2_006732
75. Kbio3_002200
76. Ninds_000309
77. 4-hydroxyquinolinium-2-carboxylate
78. Hms1736a10
79. Hms1921c20
80. Hms2269g22
81. Hms3262o13
82. Hms3266c13
83. Hms3411c03
84. Hms3675c03
85. Hms3885d20
86. 4-hydroxyquinoline-2-carboxylicacid
87. Albb-014130
88. Amy18102
89. Zinc8584773
90. Tox21_500716
91. Bbl027606
92. Bdbm50233945
93. Ccg-39280
94. Mfcd03197717
95. Pdsp1_000132
96. Pdsp2_000131
97. S4719
98. Stl294769
99. Stl301826
100. 4-hydroxy-quinoline-2-carboxylic Acid
101. Akos000118368
102. Akos000277721
103. Cs-w020664
104. Db11937
105. Gs-3763
106. Hy-w110662
107. Lp00716
108. Sb67494
109. Sb67643
110. Sdccgsbi-0050694.p003
111. Idi1_000309
112. Nsc_5280455
113. Smp1_000172
114. Ncgc00015581-01
115. Ncgc00015581-02
116. Ncgc00015581-03
117. Ncgc00015581-04
118. Ncgc00015581-05
119. Ncgc00015581-06
120. Ncgc00015581-07
121. Ncgc00015581-08
122. Ncgc00015581-09
123. Ncgc00015581-14
124. Ncgc00024505-01
125. Ncgc00024505-02
126. Ncgc00024505-03
127. Ncgc00024505-04
128. Ncgc00024505-05
129. Ncgc00024505-06
130. Ncgc00024505-07
131. Ncgc00261401-01
132. Bk166244
133. Cas_492-27-3
134. Smr000112310
135. Db-008408
136. Db-081634
137. Hy-100806
138. 4-oxo-1,4-dihydroquinoline-2-carboxylicacid
139. B6227
140. Bb 0262293
141. Cs-0168103
142. Eu-0100716
143. Ft-0670692
144. Ft-0683827
145. H0303
146. En300-13998
147. C01717
148. K 3375
149. K-8900
150. S12153
151. 593d947
152. A847277
153. Ae-641/00585057
154. Q642217
155. J-006786
156. Sr-01000075455-1
157. Sr-01000075455-3
158. Z94602408
159. 6f535706-b297-4930-a3fc-7a2823830118
| Molecular Weight | 189.17 g/mol |
|---|---|
| Molecular Formula | C10H7NO3 |
| XLogP3 | 1.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 189.042593085 g/mol |
| Monoisotopic Mass | 189.042593085 g/mol |
| Topological Polar Surface Area | 66.4 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 309 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Excitatory Amino Acid Antagonists
Drugs that bind to but do not activate excitatory amino acid receptors, thereby blocking the actions of agonists. (See all compounds classified as Excitatory Amino Acid Antagonists.)
BUILDING BLOCK


