


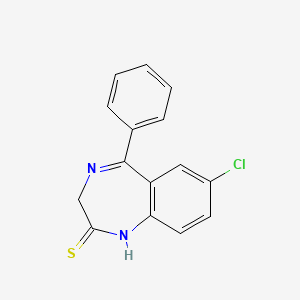

1. 4547-02-8
2. Benzp-dinitride-thio-ketone
3. Thionordazepam
4. Thionordiazepam
5. 7-chloro-5-phenyl-1,3-dihydro-1,4-benzodiazepine-2-thione
6. 7-chloro-5-phenyl-1h-benzo[e]-[1,4]diazepine-2(3h)-thione
7. Demethylsulazepam
8. 7-chloro-1,3-dihydro-5-phenyl-2h-1,4-benzodiazepine-2-thione
9. Mls000546748
10. 7-chloro-5-phenyl-1,3-dihydro-2h-1,4-benzodiazepine-2-thione
11. U953l6zt3x
12. Chembl1492697
13. 7-chloro-1,3-dihydro-5-phenyl-2h-1,4-benzodiazepin-2-thione
14. Smr000113873
15. 1,3-dihydro-7-chloro-5-phenyl-2h-1,4-benzodiazepine-2-thione
16. 7-chloranyl-5-phenyl-1,3-dihydro-1,4-benzodiazepine-2-thione
17. 2h-1,4-benzodiazepine-2-thione, 7-chloro-1,3-dihydro-5-phenyl-
18. Unii-u953l6zt3x
19. Cid_826706
20. Schembl7323716
21. Schembl11530714
22. Bdbm71907
23. Dtxsid201342681
24. Hms2342p04
25. (z)-7-chloro-5-phenyl-1h-benzo[e][1,4]diazepine-2(3h)-thione
26. Bdbm50452426
27. Mfcd02068928
28. Zinc38337185
29. Akos005067468
30. Ac-8701
31. Ds-17472
32. Db-070654
33. Cs-0151398
34. Vu0474026-1
35. A51082
36. A826812
37. Ae-641/00770055
38. 7-chloro-5phenyl-3-h-1,4-benzodiazepine-2-thione
39. Q-201825
40. 1,3-dihydro-5-phenyl-7-chloro-2h-1,4-benzodiazepine-2-thione
41. 1,3-dihydro-7chloro-5phenyl-2h-1,4-benzodiazepine-2-thione
42. 7-chloro-2,3-dihydro-5-phenyl-1h-1,4-benzodiazepine-2-thione
43. 7-chloro-5-phenyl-1,3-dihydro-[1,4]benzodiazepine-2-thione
44. 7-chloro-1,3-dihydro-5-phenyl-2h-1,4-benzenodiazepine-2-thione
45. 9-chloro-6-phenyl-2.5-diazabicyclo[5.4.0]undeca-1(7),5,8,10-tetraene-3-thione
| Molecular Weight | 286.8 g/mol |
|---|---|
| Molecular Formula | C15H11ClN2S |
| XLogP3 | 3.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 286.0331472 g/mol |
| Monoisotopic Mass | 286.0331472 g/mol |
| Topological Polar Surface Area | 56.5 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 376 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |


