

1. Acid, Isolithocholic
2. Acid, Lithocholic
3. Isolithocholic Acid
4. Lithocholate
1. 434-13-9
2. Lithocolic Acid
3. Lithocholate
4. 3alpha-hydroxy-5beta-cholan-24-oic Acid
5. 3alpha-hydroxy-5beta-cholanic Acid
6. 3alpha-hydroxycholanic Acid
7. Lithocholicacid
8. 3-alpha-hydroxycholanic Acid
9. 5beta-cholanic Acid-3alpha-ol
10. 3
11. A-hydroxy-5
12. A-cholanic Acid
13. Nci-c03861
14. 3-hydroxycholan-24-oic Acid
15. 3alpha-hydroxy-5beta-cholanoic Acid
16. (3alpha,5beta)-3-hydroxycholan-24-oic Acid
17. 3-alpha-hydroxy-5-beta-cholanic Acid
18. 3alpha-hydroxy-5beta-cholanate
19. 5-beta-cholanic Acid, 3-alpha-hydroxy-
20. Cholan-24-oic Acid, 3-hydroxy-, (3alpha,5beta)-
21. 5beta-cholan-24-oic Acid, 3alpha-hydroxy-
22. Nsc683770
23. (3-alpha,5-beta)-3-hydroxycholan-24-oic Acid
24. Chembl1478
25. 17beta-(1-methyl-3-carboxypropyl)etiocholan-3alpha-ol
26. 3.alpha.-hydroxycholanic Acid
27. 17-beta-(1-methyl-3-carboxypropyl)ethiocholan-3-alpha-ol
28. (4r)-4-[(3r,5r,8r,9s,10s,13r,14s,17r)-3-hydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl]pentanoic Acid
29. Chebi:16325
30. 5-beta-cholan-24-oic Acid, 3-alpha-hydroxy-
31. Cholan-24-oic Acid, 3-hydroxy-, (3a,5b)-
32. 5qu0i8393u
33. Nsc-683770
34. Lca
35. Litocholic Acid
36. (3beta,5beta,14beta,17alpha)-3-hydroxycholan-24-oic Acid
37. Mfcd00003682
38. (r)-4-((3r,5r,8r,9s,10s,13r,14s,17r)-3-hydroxy-10,13-dimethylhexadecahydro-1h-cyclopenta[a]phenanthren-17-yl)pentanoic Acid
39. Ccris 363
40. Hsdb 4113
41. Sr-05000000450
42. Einecs 207-099-1
43. Nsc 657956
44. Brn 3217757
45. Cholan-24-oic Acid, 3-hydroxy-, (3.alpha.,5.beta.)-
46. Unii-5qu0i8393u
47. Lithocholic-acid
48. Nsc657956
49. Nsc-657956
50. Prestwick_88
51. 4oa
52. Cas-434-13-9
53. 5beta-cholan-24-oic Acid-3alpha-ol
54. Nsc 683770
55. Lithocholic Acid,(s)
56. 4q0a
57. St069335
58. Prestwick0_000796
59. Prestwick1_000796
60. Prestwick2_000796
61. Prestwick3_000796
62. Spectrum5_002021
63. Bmse000686
64. Upcmld-dp153
65. Cid_9903
66. Lithocholic Acid, >=95%
67. Bidd:pxr0054
68. Schembl28449
69. 3a-hydroxy-5b-cholanic Acid
70. Bspbio_000932
71. Gtpl611
72. 4-10-00-00785 (beilstein Handbook Reference)
73. Mls002154006
74. 3a-hydroxy-5ss-cholanic Acid
75. Lithocholic Acid [mi]
76. Spbio_002871
77. Bpbio1_001026
78. Cholan-24-oic Acid, 3-hydroxy-, (3-alpha,5-beta)-
79. 3a-hydroxy-5b-cholan-24-oate
80. Lithocholic Acid [hsdb]
81. Dtxsid6020779
82. Upcmld-dp153:001
83. 5ss--cholan-24-oic Acid-3a-ol
84. Hms1570o14
85. Hms2097o14
86. Hms2269c14
87. Hms3714o14
88. 3a-hydroxy-5b-cholan-24-oic Acid
89. Hy-b0172
90. Zinc3918156
91. Tox21_201868
92. Tox21_302791
93. 3a-hydroxy-5ss-cholan-24-oic Acid
94. 5.beta.-cholanic Acid-3.alpha.-ol
95. Bdbm50236238
96. Lmst04010003
97. S4003
98. (3a,5b)-3-hydroxy-cholan-24-oate
99. Akos016010251
100. Lithocholic Acid [ep Impurity]
101. Ccg-220796
102. Cs-2049
103. Ds-3878
104. 3.alpha.-hydroxy-5.beta.-cholanic Acid
105. Ncgc00091272-01
106. Ncgc00091272-04
107. Ncgc00091272-06
108. Ncgc00091272-07
109. Ncgc00091272-08
110. Ncgc00256451-01
111. Ncgc00259417-01
112. (3a,5b)-3-hydroxy-cholan-24-oic Acid
113. (4s)-4-((1s,2s,11s,5r,7r,10r,14r,15r)-5-hydroxy-2,15-dimethyltetracyclo[8.7.0. 0<2,7>.0<11,15>]heptadec-14-yl)pentanoic Acid
114. 3.alpha.-hydroxy-5.beta.-cholanoic Acid
115. Nci60_028903
116. Nci60_030095
117. Smr000112168
118. 5.beta.-cholan-24-oic Acid-3.alpha.-ol
119. L0089
120. 5-.beta.-cholanic Acid, 3-.alpha.-hydroxy-
121. 3.alpha.-hydroxy-5.beta.-cholan-24-oic Acid
122. C03990
123. En300-393802
124. 5.beta.-cholan-24-oic Acid, 3.alpha.-hydroxy-
125. A872700
126. Q3323035
127. Sr-05000000450-2
128. Sr-05000000450-4
129. Sr-05000000450-5
130. Ursodeoxycholic Acid Impurity C [ep Impurity]
131. Z2216887942
132. 17-.beta.-(1-methyl-3-carboxypropyl)ethiocholan-3-.alpha.-ol
133. 3alpha-hydroxy-5beta-cholan-24-oic Acid (lithocholic Acid)
134. Cholan-24-oic Acid, 3-hydroxy-, (3-.alpha., 5-.beta.)-
135. Cholan-24-oic Acid, 3-hydroxy-, (3-alpha,5-beta)- (9ci)
136. 17.beta.-(1-methyl-3-carboxypropyl)etiocholan-3.alpha.-ol
137. Lithocholic Acid, European Pharmacopoeia (ep) Reference Standard
138. Ac268b61-0548-4391-90e9-546636926870
139. Lithocholic Acid, 50 Mug/ml In Methanol, Certified Reference Material
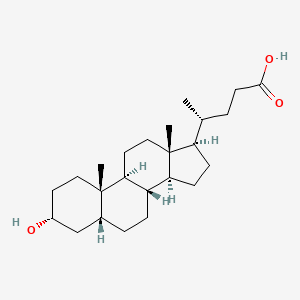
140. (4r)-4-((3r,8r,9s,10s,13r,14s,17r)-3-hydroxy-10,13-dimethylhexadecahydro-1h-cyclopenta[a]phenanthren-17-yl)pentanoic Acid
141. (4r)-4-[(1r,3as,3br,5ar,7r,9as,9bs,11ar)-7-hydroxy-9a,11a-dimethyl-hexadecahydro-1h-cyclopenta[a]phenanthren-1-yl]pentanoic Acid
142. (4r)-4-[(3r,5r,8r,9s,10s,13r,14s,17r)-3-hydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl]pentanoicacid
143. 3alpha-hydroxy-5beta-cholan-24-oic Acid 3alpha-hydroxy-5beta-cholanic Acid 3alpha-hydroxycholanic Acid 3-hydroxycholanic Acid 5beta-cholan-24-oic Acid-3alpha-ol 5beta-cholan-24-oic Acid-3a-ol 5beta-cholanic Acid-3alpha-ol Beta-cholanic Acid-3-alpha-ol Hydroycholanic Acid
1. Cas 434-13-9
2. 434-13-9
3. Cas-434-13-9
| Molecular Weight | 376.6 g/mol |
|---|---|
| Molecular Formula | C24H40O3 |
| XLogP3 | 6.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 57.5 |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 574 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Detergents
Purifying or cleansing agents, usually salts of long-chain aliphatic bases or acids, that exert cleansing (oil-dissolving) and antimicrobial effects through a surface action that depends on possessing both hydrophilic and hydrophobic properties. (See all compounds classified as Detergents.)
LITHOCHOLIC ACID (24)C(14) IS CONVERTED BY RAT LIVER HOMOGENATE INTO 3ALPHA-6BETA-DIHYDROXY-5BETA-CHOLANIC ACID, 7SIGMA-HYDROXYLATION OCCURS, HYDROXYLATION CONJUGATION WITH TAURINE & FORMATION OF 3-SULFATE ESTER CAN BE DEMONSTRATED.
BACK P, SCHERNAU-POTZI L; NAUNYN-SCHMIEDEBERG'S ARCH PHARMACOL 275 (2): 135 (1972)
LABELED LITHOCHOLATE WAS INJECTED INTO GALLSTONE PATIENTS & HEALTHY VOLUNTEERS, MAJORITY OF RADIOACTIVITY IN BILE (50-60%) WAS PRESENT AS SULFATED CONJUGATES. DEGREE OF SULFATION WAS GREATER FOR GLYCINE THAN TAURINE CONJUGATES, WHICH SUGGESTED PREFERENTIAL SULFATION OF GLYCINE CONJUGATES.
PMID:955496 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1411124 ALLAN RN ET AL; GUT 17 (6): 413 (1976)
Lithocholic Acid has known human metabolites that include 6alpha-Hydroxylithocholic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
BUILDING BLOCK


