




1. 355806-00-7
2. Rosuvastatin Tert-butyl Ester
3. Ent-rosuvastatin Tert-butyl Ester
4. 615263-60-0
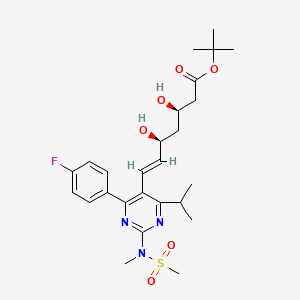
5. (3r,5s,6e)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[(methanesulfonyl) Methylamino]pyrimidin-5-yl]-3,5-dihydroxyhept-6-enoic Acid Tert-butyl Ester
6. Tert-butyl (e,3r,5s)-7-[4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-propan-2-ylpyrimidin-5-yl]-3,5-dihydroxyhept-6-enoate
7. Schembl1025729
8. Schembl1025730
9. Amy4204
10. Dtxsid10467120
11. Bcp13063
12. Mfcd08460212
13. Zinc67665366
14. Akos015841661
15. Akos015896307
16. Ac-3415
17. As-15047
18. Cs-0144285
19. A24806
20. D97190
21. 806b007
22. J-524818
23. (3r,5r)-tert-butyl Rosuvastatin (rosuvastatin Impurity)
24. (3r,5s,6e)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[(methanesulfonyl) Methylamino]-pyrimidin-5-yl]-3,5-dihydroxyhept-6-enoic Acid Tert-butyl Ester
25. (3r,5s,6e)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[(methanesulfonyl) Methylamino]pyrimidin-5-yl]-3,5-dihydroxyhept-6-enoic Acid Tert-butyl Est
26. (3r,5s,e)-tert-butyl 7-(4-(4-fluorophenyl)-6-isopropyl-2-(n-methylmethylsulfonamido)pyrimidin-5-yl)-3,5-dihydroxyhept-6-enoate
27. Rel-tert-butyl (3r,5s,e)-7-(4-(4-fluorophenyl)-6-isopropyl-2-(n-methylmethylsulfonamido)pyrimidin-5-yl)-3,5-dihydroxyhept-6-enoate
28. Rel-tert-butyl(3r,5s,e)-7-(4-(4-fluorophenyl)-6-isopropyl-2-(n-methylmethylsulfonamido)pyrimidin-5-yl)-3,5-dihydroxyhept-6-enoate
29. Tert-butyl (3r,5s,6e)-7-[4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-(propan-2-yl)pyrimidin-5-yl]-3,5-dihydroxyhept-6-enoate
30. Tert-butyl (6e)-7-{4-(4-flurophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]pyrimidin-5-yl}(3r,5s)-3,5-dihydroxyhept-6-enoate
| Molecular Weight | 537.6 g/mol |
|---|---|
| Molecular Formula | C26H36FN3O6S |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 12 |
| Exact Mass | 537.23088521 g/mol |
| Monoisotopic Mass | 537.23088521 g/mol |
| Topological Polar Surface Area | 138 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 865 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |






